Unveiling the Secrets of Acidity and Alkalinity: A Guide to the pH Scale with Common Household Items
Related Articles: Unveiling the Secrets of Acidity and Alkalinity: A Guide to the pH Scale with Common Household Items
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Unveiling the Secrets of Acidity and Alkalinity: A Guide to the pH Scale with Common Household Items. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Unveiling the Secrets of Acidity and Alkalinity: A Guide to the pH Scale with Common Household Items
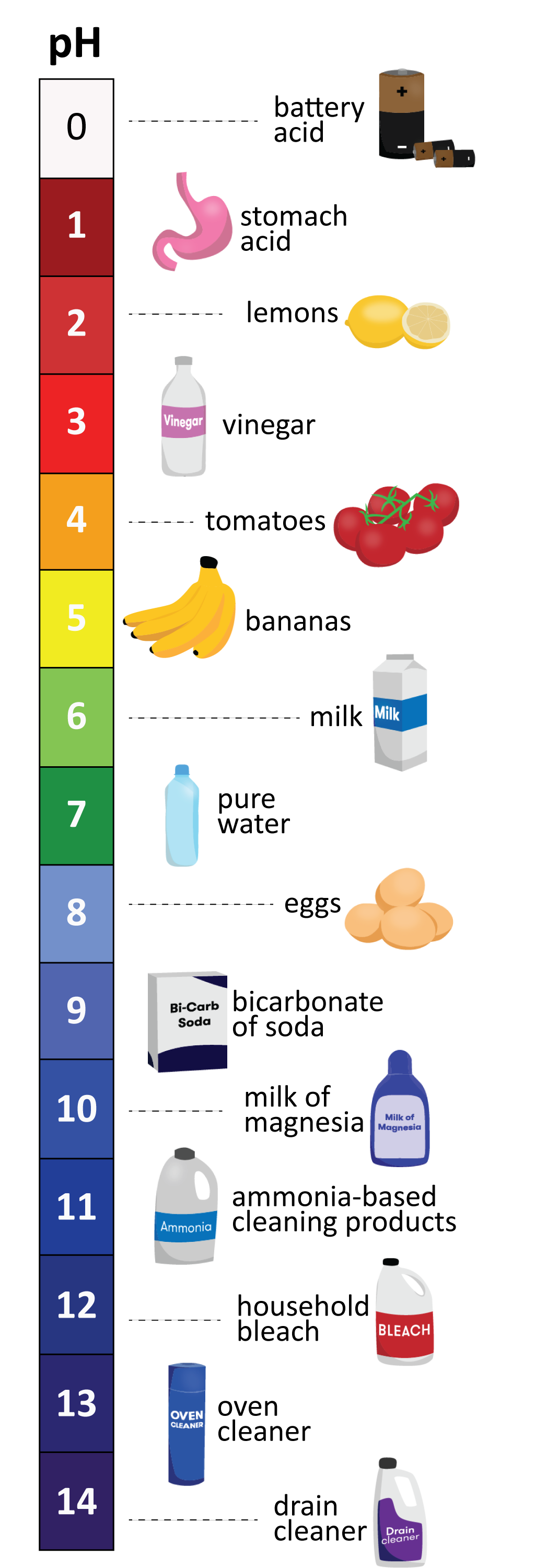
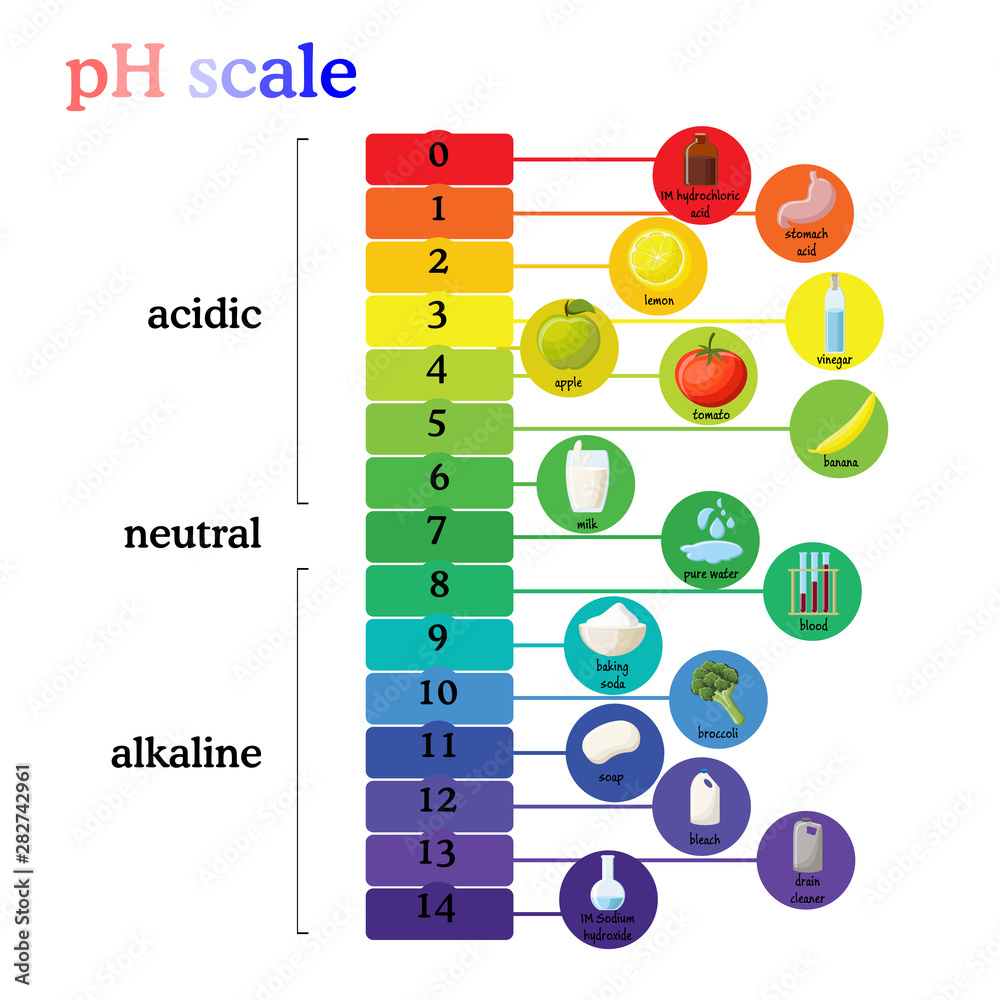
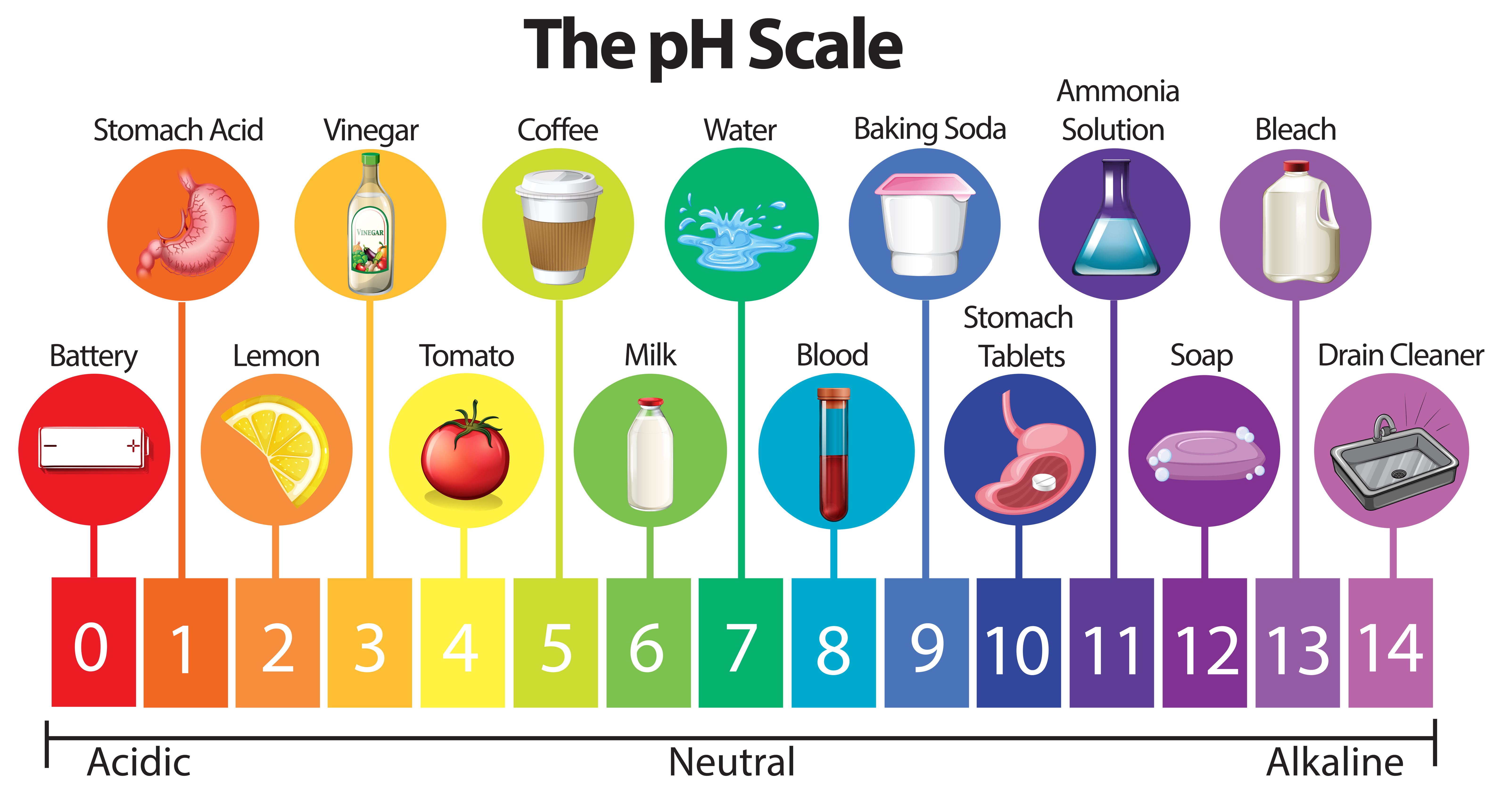
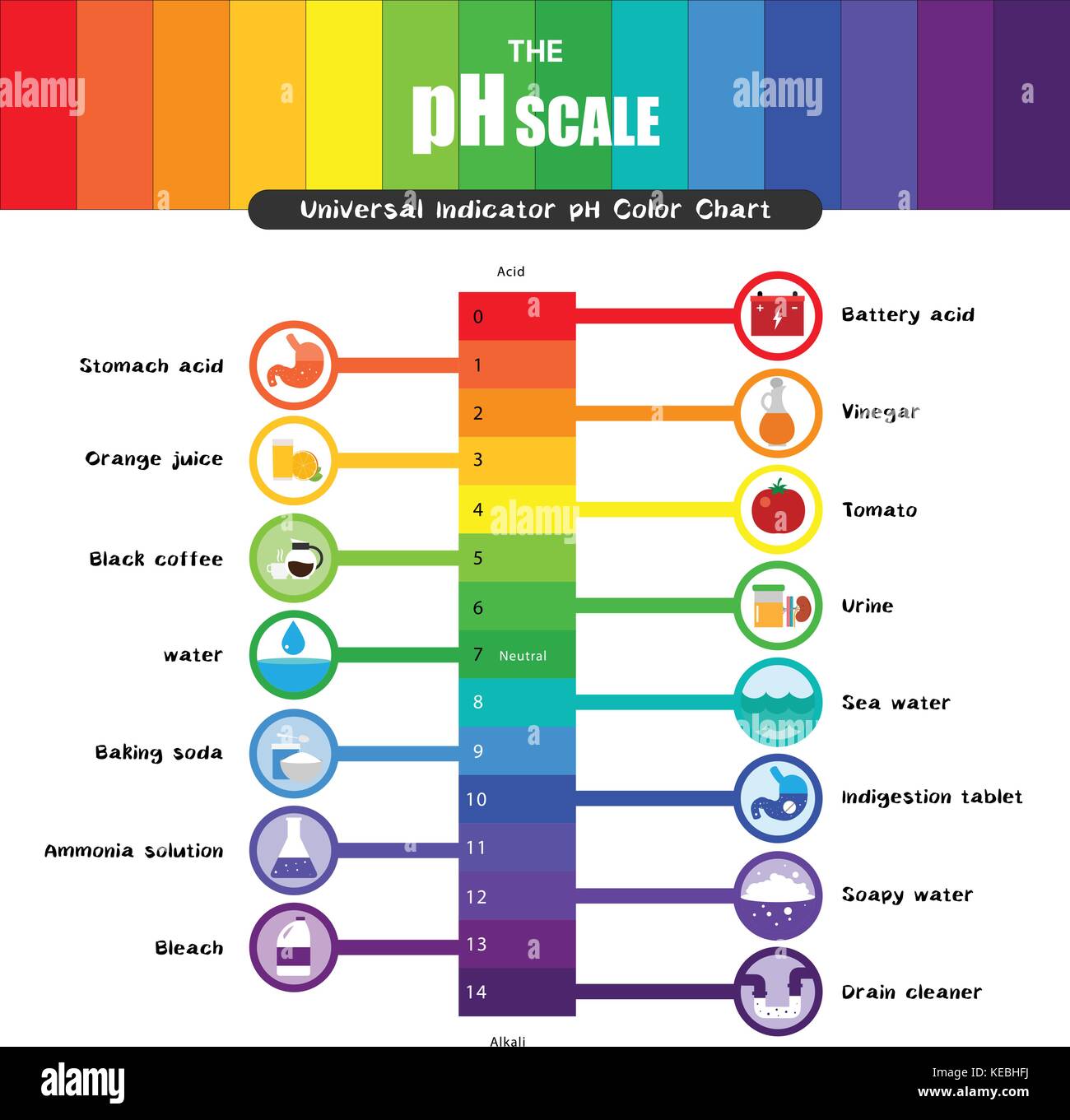
The pH scale, a numerical representation of the acidity or alkalinity of a substance, plays a vital role in various aspects of our lives, from the food we consume to the products we use. While the concept of pH may seem complex, understanding its principles and applications with everyday examples can demystify this essential scientific concept.
Understanding the pH Scale
The pH scale ranges from 0 to 14, with 7 representing a neutral solution. Values below 7 indicate acidity, increasing in strength as they approach 0, while values above 7 represent alkalinity, increasing in strength as they approach 14. The scale is logarithmic, meaning each whole number change in pH represents a tenfold change in hydrogen ion concentration.
Household Items and their pH Values
Many common household items exhibit varying levels of acidity or alkalinity. Here are some examples:
-
Acids:
- Lemon Juice: With a pH of approximately 2.0, lemon juice is highly acidic, contributing to its tart flavor.
- Vinegar: Vinegar, primarily acetic acid, has a pH of around 2.4, making it a common ingredient in pickling and salad dressings.
- Coffee: Coffee, with a pH ranging from 4.5 to 5.5, falls on the acidic side, accounting for its characteristic bitter taste.
- Orange Juice: Similar to lemon juice, orange juice boasts a pH of around 3.5, contributing to its tangy flavor.
-
Neutral:
- Pure Water: Pure water has a pH of 7.0, exhibiting neither acidic nor alkaline properties.
-
Alkaline:
- Baking Soda: Baking soda, a common household ingredient, has a pH of around 8.3, making it slightly alkaline.
- Milk of Magnesia: Milk of magnesia, often used as an antacid, has a pH of around 10.5, demonstrating its strong alkaline properties.
- Ammonia: Ammonia, a cleaning agent, has a pH of around 11.5, highlighting its highly alkaline nature.
The Importance of pH in Everyday Life
Understanding the pH of various substances is crucial in many aspects of our daily lives:
- Food and Beverage: The pH of food and beverages significantly impacts their taste, texture, and preservation. Acidic ingredients like vinegar and lemon juice are commonly used in pickling and marinades, while baking soda’s alkaline properties are vital for baking.
- Cleaning: Cleaning products often utilize the principles of pH to effectively remove dirt and grime. Acidic cleaners are often used to remove mineral deposits, while alkaline cleaners are effective in dissolving grease and oils.
- Personal Care: The pH of skin and hair products plays a critical role in maintaining their health. For example, acidic products are often used to balance the pH of skin, while alkaline shampoos are commonly used for oily hair.
- Health and Wellness: The pH of our bodies is meticulously regulated to maintain optimal health. Conditions like acid reflux and urinary tract infections can be influenced by pH imbalances.
FAQs about pH and Common Household Items:
-
Q: What is the best way to measure the pH of household items?
A: pH test strips, pH meters, and pH indicator solutions are readily available and can be used to measure the pH of household items.
-
Q: Can I use baking soda to neutralize acidic spills?
A: Yes, baking soda’s alkaline properties can neutralize acidic spills, but it’s important to wear gloves and avoid contact with eyes.
-
Q: What is the pH of soap and detergent?
A: Soap and detergent typically have a pH ranging from 8 to 11, making them slightly alkaline.
-
Q: How can I adjust the pH of my swimming pool?
A: Pool chemicals like chlorine and pH adjusters can be used to maintain the optimal pH level for safe and healthy swimming.
-
Q: What is the role of pH in soil?
A: The pH of soil significantly influences plant growth. Different plants thrive in specific pH ranges, and adjusting the pH with fertilizers and soil amendments can optimize plant health.
Tips for Utilizing pH in Everyday Life:
- Use lemon juice to brighten teeth: The citric acid in lemon juice can help remove surface stains.
- Use baking soda to deodorize refrigerators: Baking soda’s alkaline properties can absorb odors.
- Use vinegar to clean windows and mirrors: Vinegar’s acidic properties can dissolve dirt and grime.
- Use milk of magnesia to relieve heartburn: Milk of magnesia’s alkaline properties can neutralize stomach acid.
- Use pH test strips to monitor the pH of your aquarium: Maintaining the optimal pH level is crucial for fish health.
Conclusion
The pH scale, while seemingly abstract, plays a significant role in our daily lives. Understanding its principles and applications with common household items can empower us to make informed decisions about the products we use and the environment we inhabit. By embracing the concept of pH, we can unlock a deeper understanding of the world around us and make informed choices for a healthier and more sustainable future.
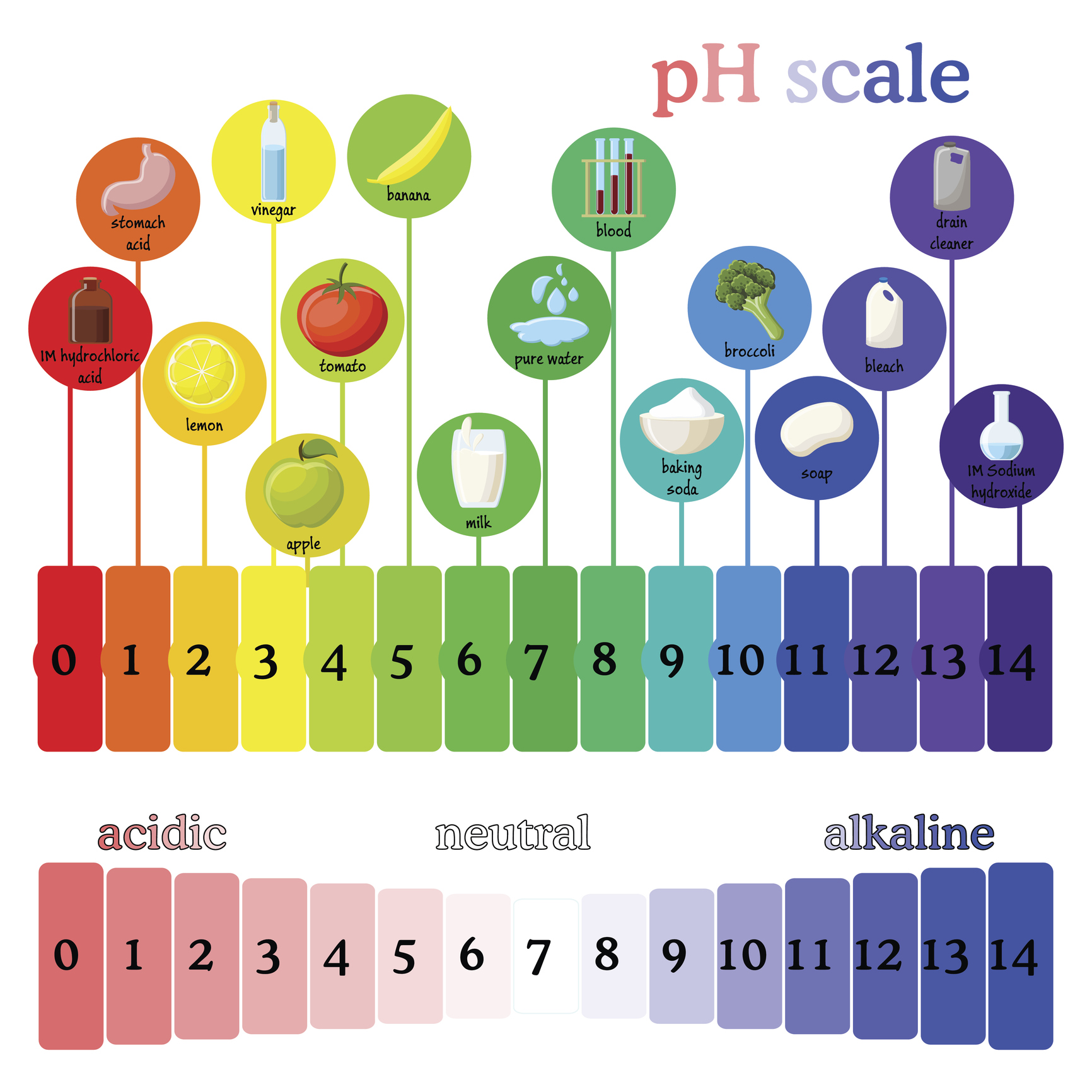

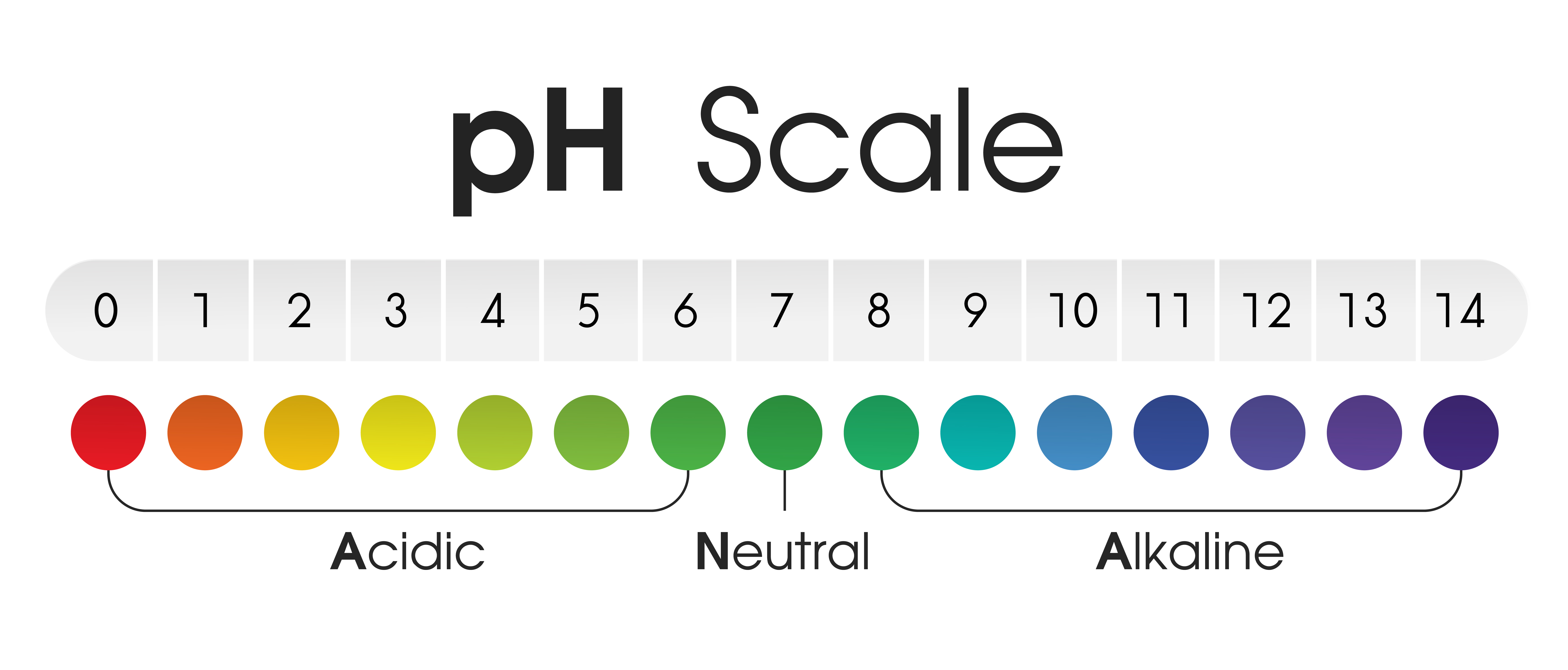
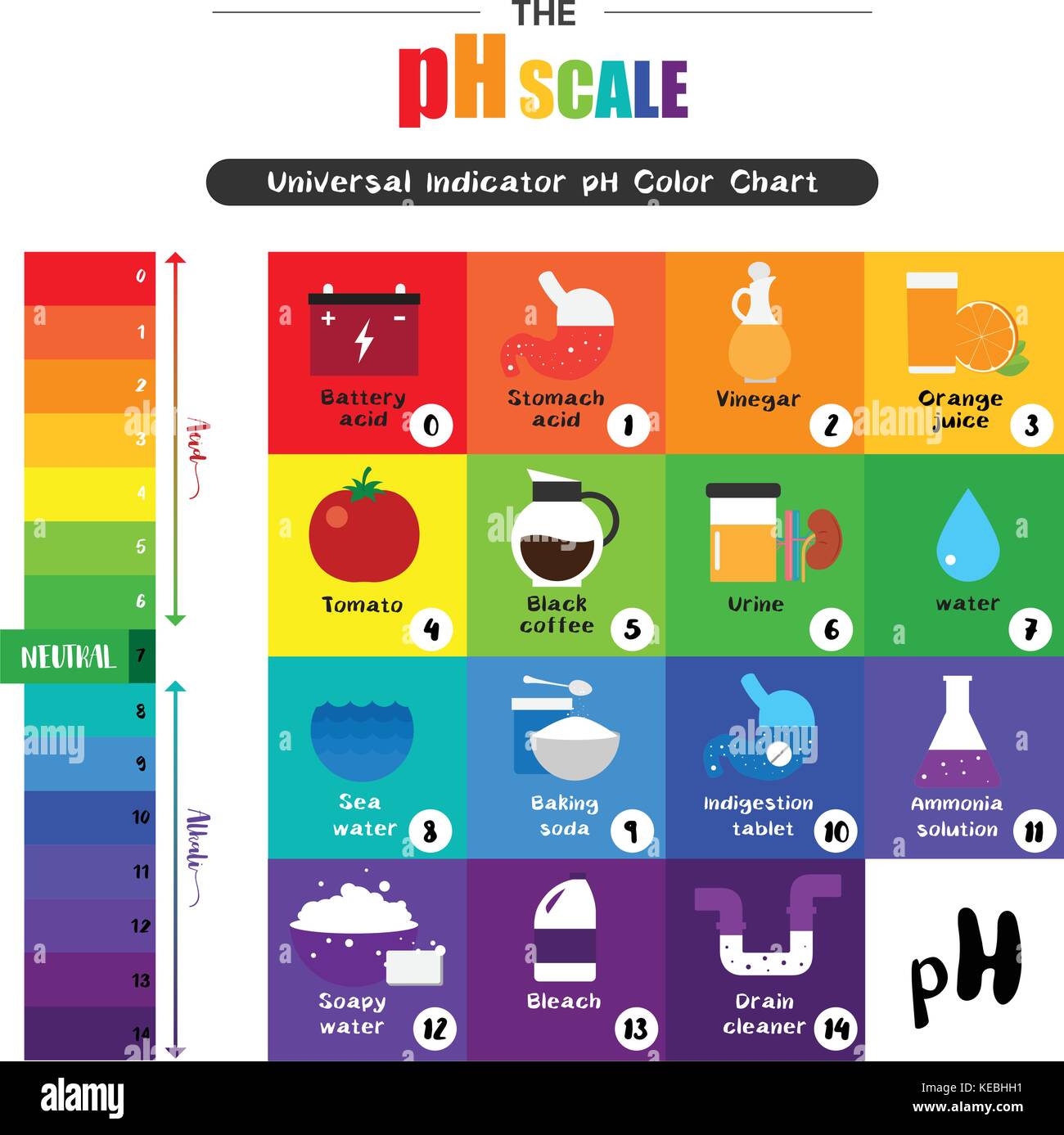

Closure
Thus, we hope this article has provided valuable insights into Unveiling the Secrets of Acidity and Alkalinity: A Guide to the pH Scale with Common Household Items. We appreciate your attention to our article. See you in our next article!