Unveiling the Hidden Chemistry of Our Homes: A Journey Through the pH Scale of Everyday Items
Related Articles: Unveiling the Hidden Chemistry of Our Homes: A Journey Through the pH Scale of Everyday Items
Introduction
With great pleasure, we will explore the intriguing topic related to Unveiling the Hidden Chemistry of Our Homes: A Journey Through the pH Scale of Everyday Items. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Unveiling the Hidden Chemistry of Our Homes: A Journey Through the pH Scale of Everyday Items

The pH scale, a numerical representation of the acidity or alkalinity of a substance, plays a crucial role in the chemical processes that shape our daily lives. It determines the efficacy of cleaning products, the safety of our food, and even the health of our skin. While the concept of pH might seem abstract, it is deeply embedded in the everyday objects and activities that surround us. This article delves into the fascinating world of pH, exploring its relevance to common household items and providing insights into its significance in our lives.
The pH Scale: A Guide to Acidity and Alkalinity
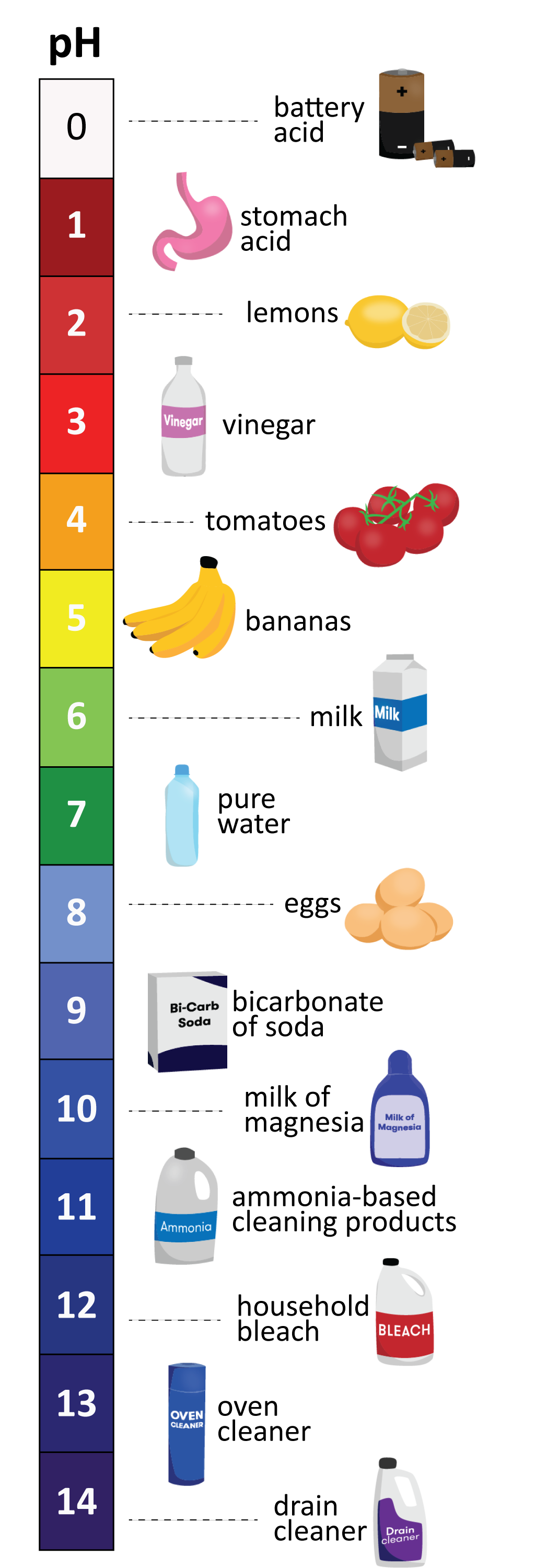
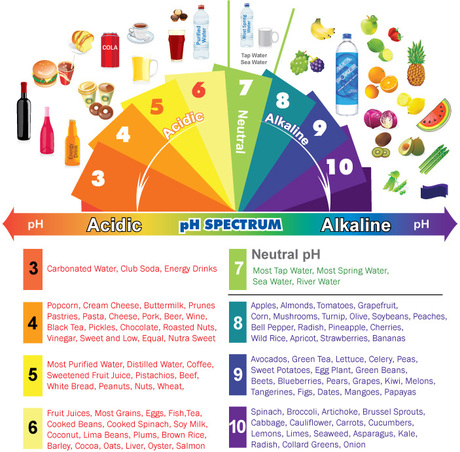
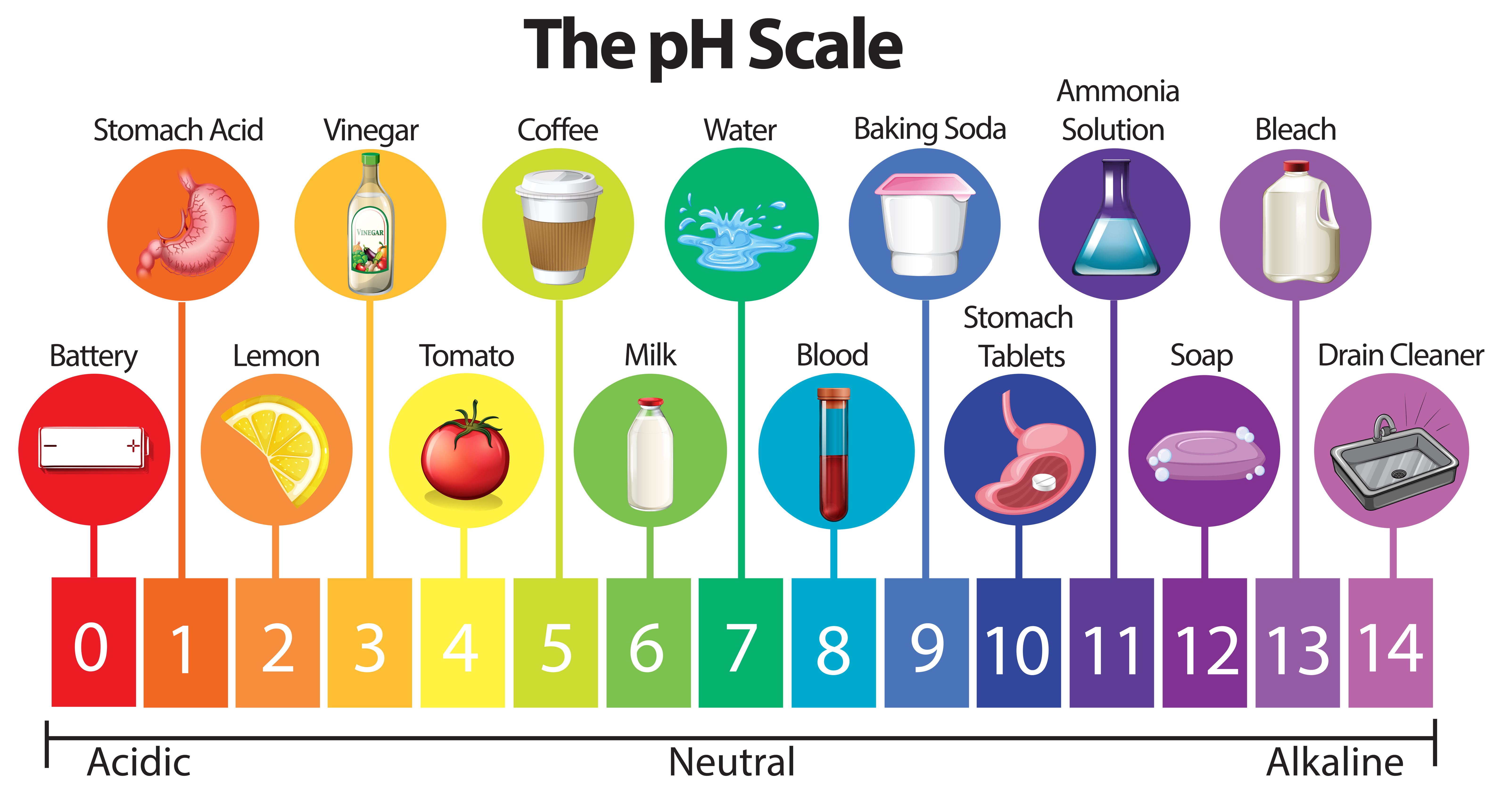
The pH scale, ranging from 0 to 14, measures the concentration of hydrogen ions (H+) in a solution. A pH value of 7 represents neutral, indicating an equal balance of H+ and hydroxide ions (OH-). Values below 7 indicate acidity, with lower numbers signifying greater acidity. Conversely, values above 7 represent alkalinity, with higher numbers signifying greater alkalinity.
Household Items and Their pH Spectrum
The pH of household items varies significantly, influencing their properties and applications. Here is a glimpse into the pH spectrum of common household items:
Acids (pH < 7):
- Vinegar (pH 2.4-3.4): A versatile household staple, vinegar is a weak acid primarily used for cleaning, deodorizing, and food preservation. Its acidity helps dissolve mineral deposits, remove grease, and neutralize unpleasant odors.
- Lemon Juice (pH 2.0-2.4): A natural acid with a tart flavor, lemon juice is often used in cooking and cleaning. Its acidity aids in tenderizing meat, brightening fabrics, and removing stains.
- Tomato Juice (pH 4.0-4.4): With its distinctive tangy taste, tomato juice is a popular beverage. Its acidity contributes to its flavor and can be used in various culinary applications.
- Coffee (pH 4.5-5.5): A beloved beverage, coffee’s acidity contributes to its unique flavor and aroma. However, its acidity can also cause stomach discomfort for some individuals.
- Milk (pH 6.4-6.8): While technically slightly acidic, milk is considered close to neutral. Its acidity contributes to its characteristic taste and contributes to its preservation.
Neutral (pH 7):
- Pure Water (pH 7): Water, in its purest form, is neutral, neither acidic nor alkaline. It serves as a vital solvent and plays a crucial role in various biological processes.
- Baking Soda (pH 8.3): Although often referred to as baking soda, sodium bicarbonate is actually a weak base. It is commonly used in baking, cleaning, and as an antacid.
Alkaline (pH > 7):
- Soap (pH 9-11): Soaps are typically alkaline, with their pH depending on the specific ingredients. Their alkalinity helps break down grease and dirt, making them effective cleaning agents.
- Bleach (pH 12-13): A strong oxidizing agent, bleach is highly alkaline and used for disinfecting and whitening purposes. Its alkalinity allows it to kill bacteria and remove stains.
- Ammonia (pH 11-12): A colorless gas with a pungent odor, ammonia is commonly used in cleaning products. Its alkalinity makes it an effective grease cutter and stain remover.
- Baking Powder (pH 8.5-9.0): Similar to baking soda, baking powder is a weak base. It is primarily used as a leavening agent in baking, helping create a fluffy texture.
The Significance of pH in Everyday Life
Understanding the pH of household items is crucial for various reasons:
- Cleaning Efficacy: The effectiveness of cleaning products is often determined by their pH. Acidic cleaners are better at dissolving mineral deposits and removing stains, while alkaline cleaners are more effective at breaking down grease and disinfecting surfaces.
- Food Safety: The pH of food plays a vital role in its preservation and safety. Acidic foods, like vinegar and lemon juice, inhibit the growth of harmful bacteria, contributing to their extended shelf life.
- Personal Care: The pH of our skin and hair is slightly acidic, with a typical range of 4.5-5.5. Maintaining this pH balance is crucial for healthy skin and hair. Using products with pH levels close to our natural skin and hair pH can help prevent irritation and maintain their health.
- Environmental Impact: The pH of water bodies can significantly impact aquatic life. Acid rain, caused by the release of acidic pollutants into the atmosphere, can lower the pH of lakes and rivers, harming fish and other aquatic organisms.
FAQs About the pH of Household Items:
Q1: Can I use vinegar and bleach together?
A: No, mixing vinegar and bleach is extremely dangerous. The reaction produces toxic chlorine gas, which can cause respiratory problems and even death.
Q2: What is the best pH for cleaning my bathroom?
A: A slightly acidic cleaner, with a pH around 4-5, is generally effective for removing mineral deposits and soap scum from bathroom surfaces.
Q3: What is the best pH for washing dishes?
A: A slightly alkaline detergent, with a pH around 8-9, is generally effective for removing grease and food particles from dishes.
Q4: How can I adjust the pH of my soil?
A: The pH of soil can be adjusted by adding acidic or alkaline materials. For acidic soils, adding lime will increase the pH, while for alkaline soils, adding sulfur or aluminum sulfate will decrease the pH.
Q5: What is the best pH for my hair?
A: The optimal pH for hair is slightly acidic, around 4.5-5.5. Shampoos and conditioners with a pH within this range can help maintain hair health and prevent damage.
Tips for Using pH Knowledge in Everyday Life:
- Read Labels: Always check the pH of cleaning products and personal care items to ensure they are suitable for your needs.
- Test Your Soil: Regularly test the pH of your garden soil to ensure optimal conditions for plant growth.
- Use Natural Remedies: Vinegar and baking soda are versatile natural cleaning agents with varying pH levels, offering safe and effective alternatives to harsh chemicals.
- Maintain Skin and Hair Health: Choose personal care products with pH levels close to your skin and hair’s natural pH to maintain their health and prevent irritation.
- Be Aware of Environmental Impact: Consider the pH of cleaning products and their potential impact on the environment. Choose eco-friendly options whenever possible.
Conclusion:
The pH scale, while often hidden from view, plays a critical role in our daily lives. Understanding the pH of household items empowers us to make informed decisions about cleaning, food safety, personal care, and environmental protection. By appreciating the delicate balance of acidity and alkalinity, we can navigate the chemical world around us with greater awareness and responsibility.



Closure
Thus, we hope this article has provided valuable insights into Unveiling the Hidden Chemistry of Our Homes: A Journey Through the pH Scale of Everyday Items. We thank you for taking the time to read this article. See you in our next article!