Unveiling the Hidden Acidity and Alkalinity of Everyday Items: A Comprehensive Guide to the pH Scale
Related Articles: Unveiling the Hidden Acidity and Alkalinity of Everyday Items: A Comprehensive Guide to the pH Scale
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to Unveiling the Hidden Acidity and Alkalinity of Everyday Items: A Comprehensive Guide to the pH Scale. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Unveiling the Hidden Acidity and Alkalinity of Everyday Items: A Comprehensive Guide to the pH Scale

The pH scale, a numerical representation of the acidity or alkalinity of a solution, is a fundamental concept in chemistry with wide-ranging implications in our daily lives. It serves as a powerful tool for understanding the chemical nature of substances we encounter regularly, from household cleaning products to the food we consume. This guide delves into the pH scale, exploring its significance and providing a comprehensive overview of the pH values of common items we interact with on a daily basis.
Understanding the pH Scale: A Numerical Representation of Acidity and Alkalinity
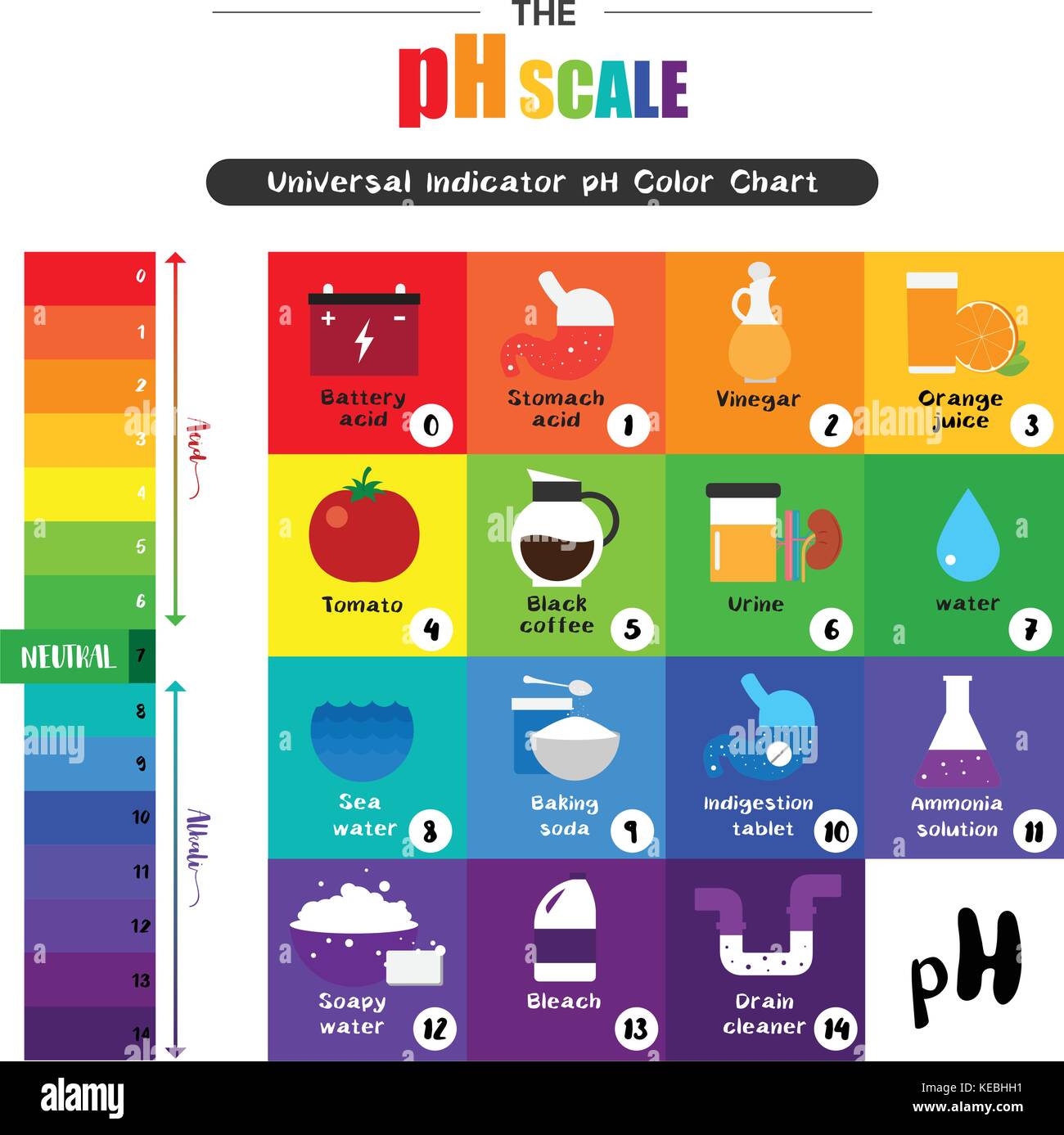
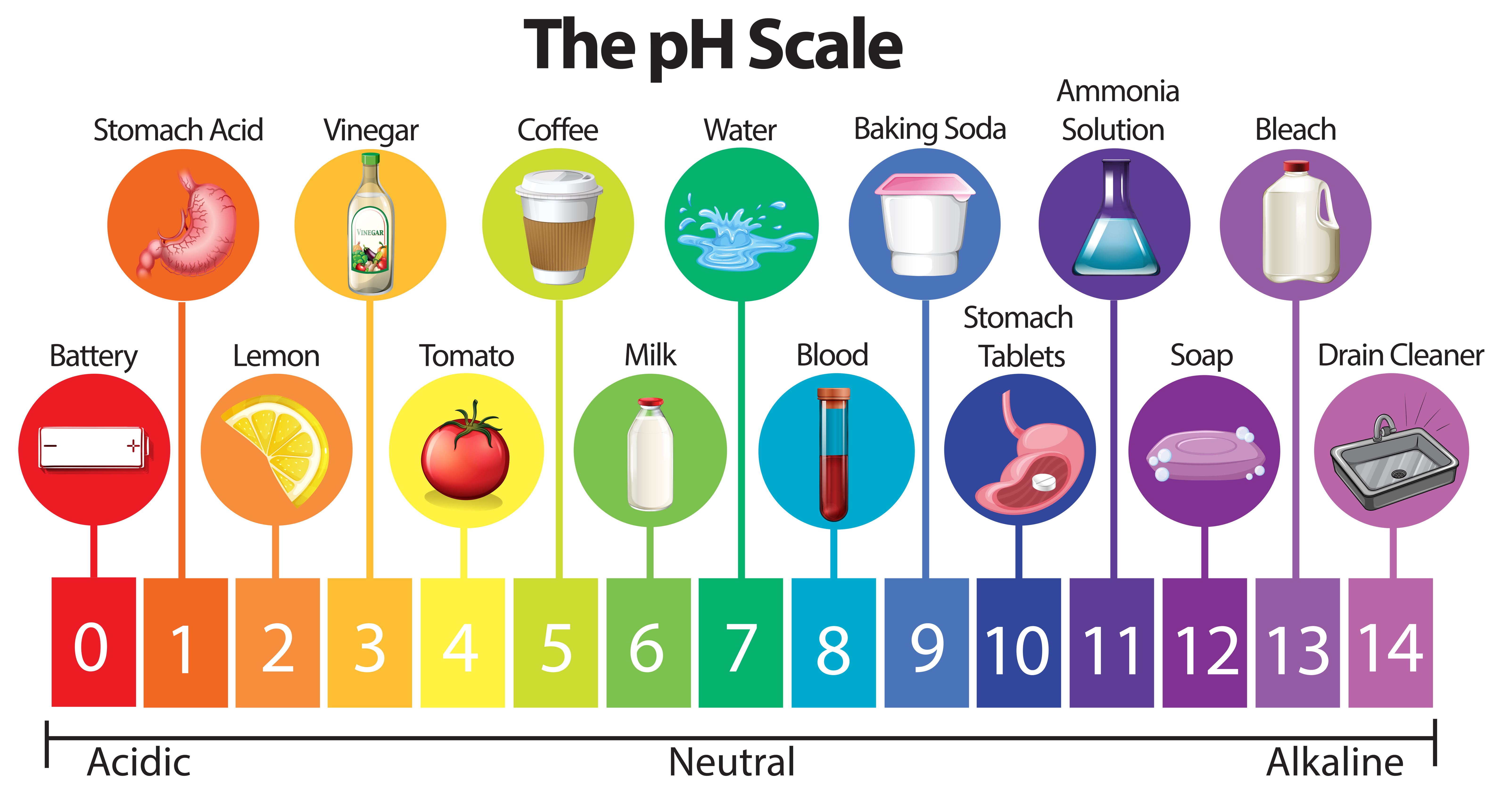
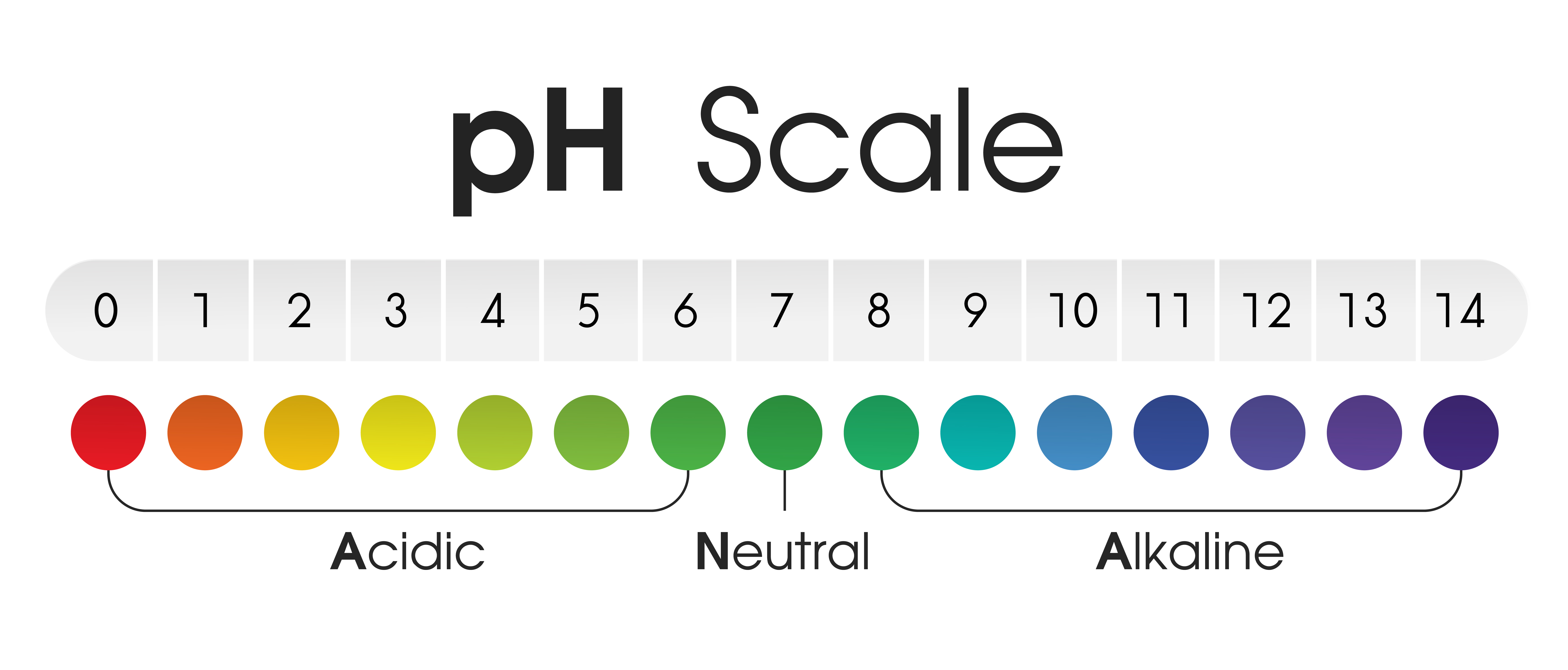
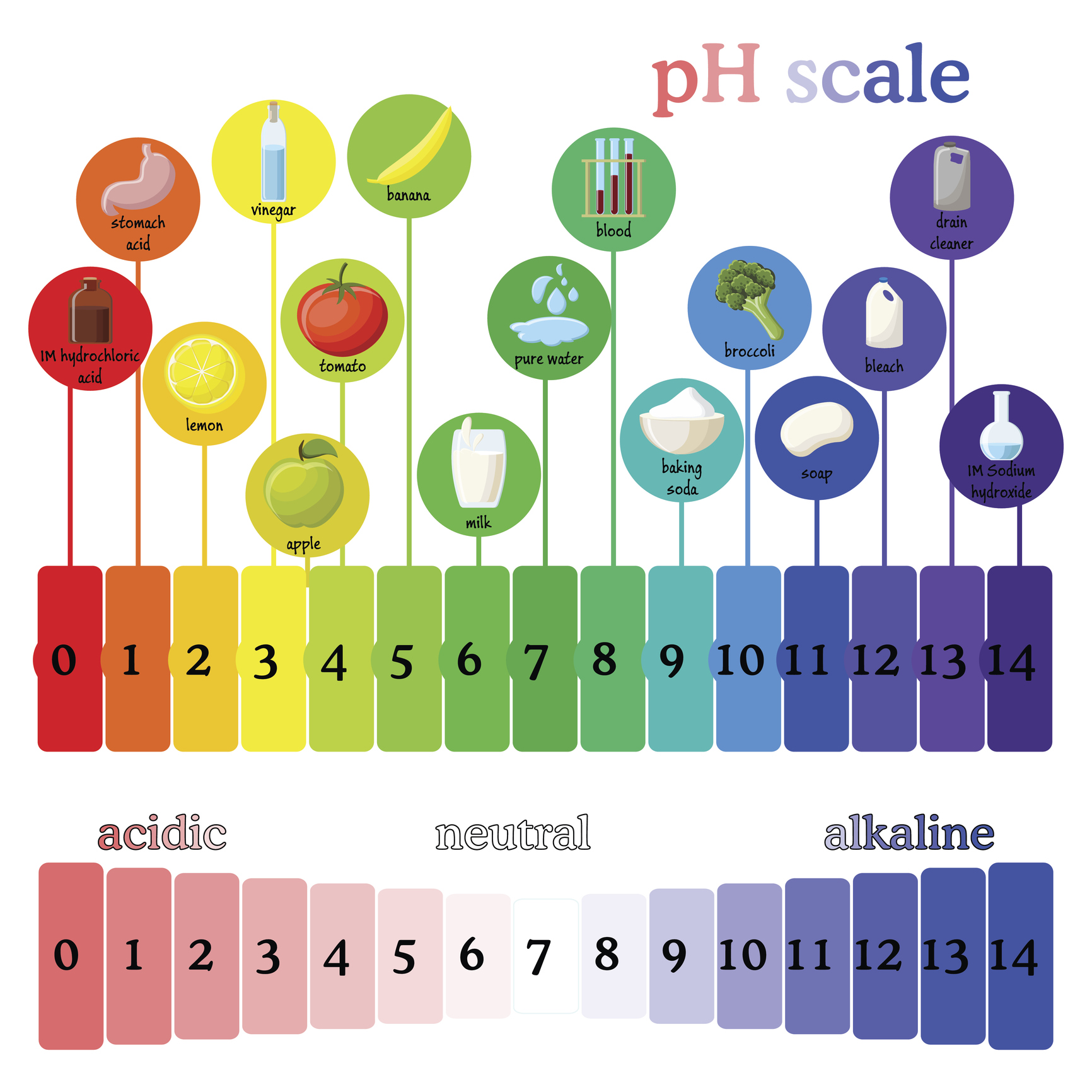
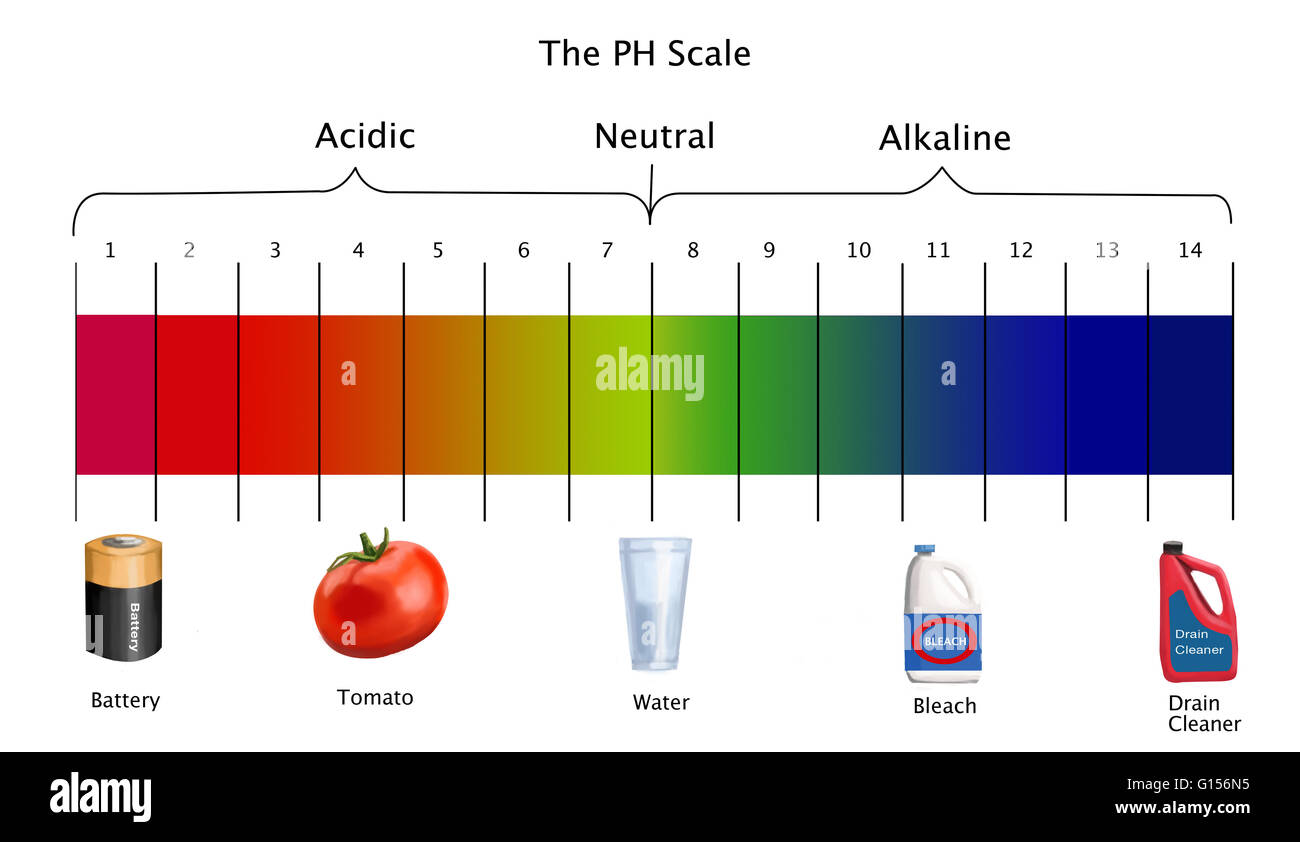
The pH scale, ranging from 0 to 14, quantifies the concentration of hydrogen ions (H+) in a solution. A lower pH value indicates a higher concentration of H+ ions, signifying a more acidic solution. Conversely, a higher pH value denotes a lower concentration of H+ ions, indicating a more alkaline (basic) solution. A pH of 7 represents a neutral solution, with an equal concentration of H+ and hydroxide ions (OH-).
The Importance of pH in Everyday Life: A Multifaceted Impact
The pH scale plays a critical role in various aspects of our daily lives. Understanding the pH of different substances allows us to:
- Maintain Optimal Health: The pH of our bodies, particularly our blood, is meticulously regulated within a narrow range (slightly alkaline). Deviation from this range can lead to various health problems.
- Ensure Safe and Effective Cleaning: The pH of cleaning products influences their effectiveness in removing dirt and grime. Acidic cleaners are often used for removing mineral deposits, while alkaline cleaners are more effective against grease and oils.
- Enhance Food Preservation and Flavor: The pH of food influences its taste, texture, and shelf life. For instance, acidic ingredients like vinegar are used for pickling, while baking soda (alkaline) is employed in baking for leavening.
- Optimize Plant Growth: Plants thrive in specific pH ranges, and understanding their soil pH allows for targeted adjustments through fertilizers or amendments to enhance growth.
- Protect the Environment: pH plays a crucial role in maintaining the ecological balance of water bodies. Acid rain, for instance, can significantly impact aquatic life by lowering the pH of lakes and rivers.
Exploring the pH of Common Items: A Journey Through Everyday Acidity and Alkalinity
Let’s delve into the pH values of various common items, providing insights into their chemical nature and their implications in our lives:
Household Products:
- Vinegar: A common household staple, vinegar typically has a pH ranging from 2 to 3, making it acidic. This acidity is the reason it’s effective in cleaning, removing mineral deposits, and preserving food.
- Lemon Juice: Similar to vinegar, lemon juice is acidic with a pH of around 2. It’s widely used in cooking, cleaning, and as a natural disinfectant.
- Baking Soda: Baking soda, a common leavening agent, has a pH of around 9, making it alkaline. It’s also used for cleaning, deodorizing, and neutralizing acidic substances.
- Dish Soap: Dish soap typically has a pH of around 7, making it slightly alkaline. This alkalinity aids in breaking down grease and oils, making it effective for cleaning dishes.
- Bleach: Bleach is a powerful disinfectant with a pH of around 12, making it strongly alkaline. Its high alkalinity allows it to effectively kill bacteria and viruses.
Food and Beverages:
- Coffee: Coffee is slightly acidic, with a pH ranging from 4 to 5. The acidity contributes to its characteristic flavor.
- Tea: Black tea is slightly acidic, with a pH of around 5. Green tea, on the other hand, is less acidic with a pH of around 6.
- Milk: Milk is slightly acidic, with a pH of around 6.5.
- Orange Juice: Orange juice is acidic, with a pH of around 3.5. This acidity is responsible for its tangy flavor.
- Tomato Juice: Tomato juice is also acidic, with a pH of around 4. This acidity gives it its characteristic flavor.
Personal Care Products:
- Shampoo: Shampoos can have a wide range of pH values, depending on their formulation. Many shampoos are slightly acidic, with a pH of around 5, to help close hair cuticles and reduce frizz.
- Conditioner: Conditioners are typically more acidic than shampoos, with a pH of around 4. This acidity helps to smooth and detangle hair.
- Soap: Soap is generally alkaline, with a pH of around 9. This alkalinity helps to break down dirt and grease.
Environmental Factors:
- Rainwater: Rainwater is naturally slightly acidic, with a pH of around 5.6. This acidity is due to the absorption of carbon dioxide from the atmosphere.
- Seawater: Seawater is slightly alkaline, with a pH of around 8.1. This alkalinity is due to the presence of dissolved minerals.
- Soil: Soil pH can vary widely depending on its composition and location. Most plants thrive in a slightly acidic to neutral pH range (6 to 7).
FAQs by pH Scale of Common Items
Q: What is the pH of human blood?
A: The pH of human blood is meticulously regulated within a narrow range of 7.35 to 7.45, making it slightly alkaline.
Q: What is the pH of stomach acid?
A: Stomach acid is highly acidic, with a pH of around 1 to 3. This acidity is essential for digestion.
Q: What is the pH of urine?
A: The pH of urine can vary depending on factors such as diet and hydration. It typically ranges from 4.5 to 8.
Q: What is the pH of saliva?
A: Saliva is slightly acidic, with a pH of around 6.5. This acidity helps to break down food and protect against bacteria.
Q: What is the pH of tap water?
A: The pH of tap water can vary depending on the source. It typically ranges from 6 to 8.
Tips by pH Scale of Common Items
- Use vinegar to clean mineral deposits: Vinegar’s acidity makes it effective for removing mineral deposits from kettles, showers, and other surfaces.
- Use baking soda to neutralize acidic spills: Baking soda’s alkalinity neutralizes acids, making it useful for cleaning up spills from citrus fruits or vinegar.
- Adjust soil pH for optimal plant growth: Test your soil pH and amend it with fertilizers or lime to achieve the optimal range for your plants.
- Choose pH-balanced shampoos for healthy hair: Select shampoos with a pH of around 5 to help maintain healthy hair and scalp.
- Monitor the pH of your swimming pool: Maintain the pH of your swimming pool within a safe range (7.2 to 7.6) to prevent skin irritation and ensure proper sanitation.
Conclusion by pH Scale of Common Items
The pH scale, a simple yet powerful tool, provides a window into the chemical nature of substances we encounter every day. Understanding the pH of common items allows us to make informed decisions regarding their use, optimize their effectiveness, and ensure their safety. From maintaining optimal health to promoting environmental sustainability, the pH scale plays a vital role in shaping our lives. By appreciating its significance and utilizing the knowledge it provides, we can navigate the world of acidity and alkalinity with greater awareness and informed choices.



Closure
Thus, we hope this article has provided valuable insights into Unveiling the Hidden Acidity and Alkalinity of Everyday Items: A Comprehensive Guide to the pH Scale. We hope you find this article informative and beneficial. See you in our next article!