The Science of Luminescence: Unveiling the Secrets of Materials that Glow Under Black Light
Related Articles: The Science of Luminescence: Unveiling the Secrets of Materials that Glow Under Black Light
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to The Science of Luminescence: Unveiling the Secrets of Materials that Glow Under Black Light. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The Science of Luminescence: Unveiling the Secrets of Materials that Glow Under Black Light

The captivating phenomenon of materials glowing under black light, often referred to as fluorescence, has captivated the human imagination for centuries. This captivating display of light, seemingly conjured from the darkness, is a testament to the intricate interplay between matter and energy, revealing a world of hidden colors and vibrant hues.
Understanding the Physics of Fluorescence
At the heart of this phenomenon lies the process of fluorescence, a form of luminescence where certain materials absorb ultraviolet (UV) radiation and then re-emit this energy as visible light. The key to this transformation lies in the electronic structure of the material.
When a UV photon strikes an atom or molecule within a fluorescent material, it excites an electron, elevating it to a higher energy level. This excited state is inherently unstable, and the electron quickly seeks to return to its ground state, releasing the excess energy in the form of a photon. The emitted photon’s wavelength, and thus its color, depends on the energy difference between the excited and ground states.
Key Players in the Fluorescent Symphony: Materials and Their Properties
The materials that exhibit fluorescence are diverse and multifaceted, each possessing unique properties that influence their luminescent behavior. Here, we delve into the key characteristics that govern a material’s ability to glow under black light:
-
Chemical Composition: The chemical composition of a material plays a pivotal role in determining its fluorescent properties. Organic molecules, particularly those containing aromatic rings, are known for their strong fluorescence. For example, the presence of conjugated systems, where alternating single and double bonds create a delocalized electron cloud, enhances the absorption of UV radiation and facilitates fluorescence. Examples include dyes like fluorescein and rhodamine, which emit bright green and red fluorescence, respectively.
-
Molecular Structure: The arrangement of atoms within a molecule also influences its fluorescence. Rigid molecules, with limited vibrational and rotational freedom, tend to exhibit stronger fluorescence compared to flexible molecules. This is because rigid structures minimize energy loss through non-radiative pathways, increasing the likelihood of radiative emission.
-
Presence of Chromophores: Chromophores, specific functional groups within a molecule that absorb light, are crucial for fluorescence. These groups often contain conjugated systems, which enhance the absorption of UV radiation. The type of chromophore dictates the wavelength of light absorbed and the resulting color of fluorescence.
-
Solvent Effects: The solvent in which a fluorescent molecule is dissolved can significantly influence its fluorescence intensity and lifetime. Solvents with high polarity can interact with the excited state of the molecule, leading to quenching, a process where the excited state is deactivated without emitting light.
-
Temperature: Temperature also plays a role in fluorescence. Higher temperatures generally lead to increased molecular vibrations and collisions, which can enhance non-radiative decay pathways, thereby reducing fluorescence intensity.
A Spectrum of Fluorescence: Exploring Different Materials and Their Applications
The world of fluorescent materials extends far beyond the realm of laboratory experiments, finding diverse applications across various industries. Here, we explore some prominent examples:
1. Fluorescent Dyes and Pigments:
- Applications: Fluorescent dyes and pigments are widely employed in various fields, including textiles, plastics, and inks. They impart vibrant colors to fabrics, add visual appeal to plastics, and provide security features on documents and banknotes.
- Examples: Fluorescein, rhodamine, and coumarin dyes are commonly used in textile dyeing and printing, while fluorescent pigments like cadmium sulfide and zinc sulfide are incorporated into plastics and paints.
2. Fluorescent Proteins:
- Applications: Fluorescent proteins are invaluable tools in biological research, enabling scientists to visualize cellular processes, track protein movement, and study gene expression.
- Examples: Green fluorescent protein (GFP), a protein isolated from jellyfish, has revolutionized biological imaging. Other fluorescent proteins, such as red fluorescent protein (RFP) and blue fluorescent protein (BFP), offer a wider range of colors for multi-color imaging.
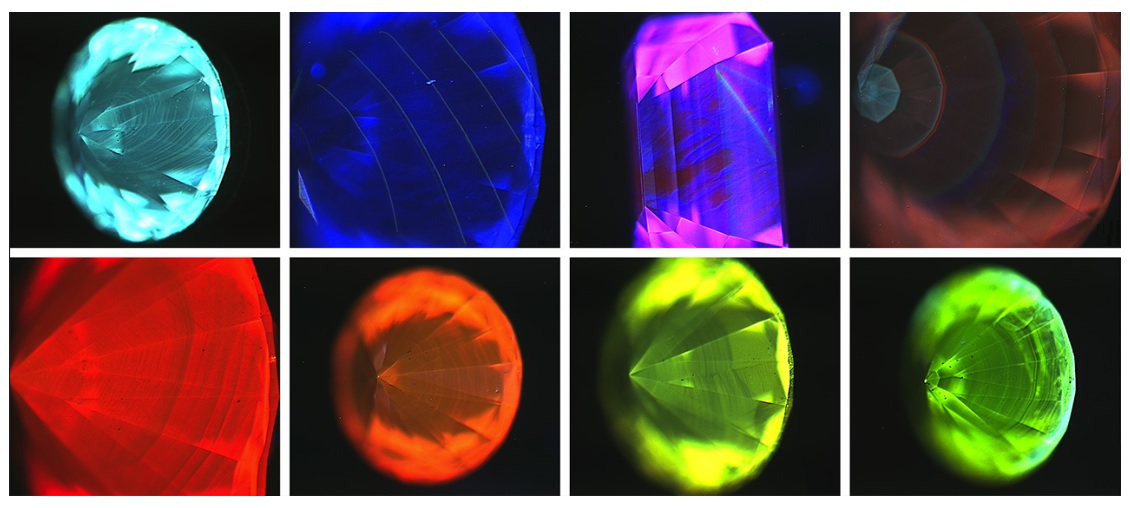
3. Fluorescent Minerals:
- Applications: Fluorescent minerals are prized by collectors for their captivating displays of color under UV light. They also serve as indicators of mineral composition and geological formations.
- Examples: Fluorite, calcite, and scheelite are among the most common fluorescent minerals, exhibiting a range of colors from blue to green to yellow.
4. Fluorescent Markers and Indicators:
- Applications: Fluorescent markers and indicators are used in various analytical techniques, including chromatography, electrophoresis, and flow cytometry. They facilitate the detection and quantification of specific molecules or biological entities.
- Examples: Fluorescent dyes like ethidium bromide are used to stain DNA in gel electrophoresis, while fluorescent antibodies are employed in flow cytometry for cell sorting and analysis.
5. Fluorescent Lighting:
- Applications: Fluorescent lamps have become ubiquitous in lighting applications, offering energy efficiency and long lifespan compared to traditional incandescent bulbs.
- Examples: Compact fluorescent lamps (CFLs) and linear fluorescent tubes are widely used in homes, offices, and public spaces.
6. Security Applications:
- Applications: Fluorescent materials play a vital role in security applications, providing covert markings and authentication features.
- Examples: Fluorescent inks and pigments are used in banknotes, passports, and other security documents to prevent counterfeiting.
7. Medical Imaging:
- Applications: Fluorescent dyes and probes are used in medical imaging techniques like fluorescence microscopy and fluorescence-guided surgery. They allow for real-time visualization of tissues, cells, and biological processes.
- Examples: Fluorescent dyes like fluorescein are used to visualize blood vessels during surgery, while fluorescent probes are employed to detect tumors and monitor disease progression.
FAQs: Demystifying the World of Fluorescent Materials
1. What is the difference between fluorescence and phosphorescence?
Fluorescence and phosphorescence are both forms of luminescence, but they differ in the mechanism of light emission. In fluorescence, the excited state decays rapidly, typically within nanoseconds, leading to immediate light emission. In phosphorescence, the excited state is metastable, meaning it persists for a longer duration, typically milliseconds to seconds, before decaying and emitting light. This delay in light emission is what gives phosphorescent materials their "afterglow" effect.
2. How can I identify fluorescent materials?
The easiest way to identify a fluorescent material is to expose it to UV light. If the material glows under UV light, it is fluorescent. However, it’s important to note that not all materials that glow under UV light are fluorescent. Some materials may exhibit phosphorescence or chemiluminescence, which are different phenomena.
3. What are some common uses of fluorescent materials in everyday life?
Fluorescent materials are found in various everyday objects, including:
- Highlighters: The bright colors of highlighters are due to fluorescent dyes.
- Detergent: Fluorescent dyes are added to detergents to make them appear brighter and whiter.
- Clothing: Some clothing items, particularly white shirts, contain fluorescent dyes to enhance their brightness.
- Safety vests: Fluorescent vests worn by construction workers and cyclists enhance their visibility in low-light conditions.
4. Are fluorescent materials harmful to humans?
The safety of fluorescent materials depends on the specific material and its intended use. Some fluorescent dyes and pigments can be toxic if ingested or absorbed through the skin. However, most fluorescent materials used in everyday products are considered safe when used as intended.
5. What are the future prospects for fluorescent materials?
The field of fluorescent materials is continuously evolving, with new discoveries and applications emerging regularly. Future research focuses on developing:
- More efficient fluorescent materials: Researchers are exploring new materials with higher fluorescence quantum yields, leading to brighter and more efficient luminescence.
- Fluorescent materials with specific properties: Scientists are developing fluorescent materials with tailored properties, such as specific excitation and emission wavelengths, for specific applications.
- Fluorescent materials for advanced technologies: Fluorescent materials are being investigated for applications in optoelectronics, sensing, and nanotechnology.
Tips for Utilizing Fluorescent Materials
- Protect Fluorescent Materials from UV Degradation: Fluorescent materials can degrade over time when exposed to UV light. Store them in dark, cool environments to preserve their fluorescence.
- Consider the Application: Select fluorescent materials with properties suited to the specific application. For example, for high-visibility applications, choose materials with high fluorescence quantum yields and appropriate excitation wavelengths.
- Ensure Safety: When handling fluorescent materials, follow safety guidelines and wear appropriate protective gear.
Conclusion: A World Illuminated by Fluorescence
The phenomenon of fluorescence, while seemingly simple, unveils a complex interplay between light, matter, and energy. This captivating display of colors, hidden in plain sight, continues to inspire scientists and artists alike, driving innovation across diverse fields. From illuminating our homes and workplaces to revealing the intricacies of life at the molecular level, fluorescent materials are a testament to the power of light and the endless possibilities it offers. The future holds exciting prospects for this versatile class of materials, promising further advancements in our understanding of the world around us and paving the way for novel technologies that harness the power of fluorescence.

/flasks-with-glowing-liquids-520120820-594044535f9b58d58a548082.jpg)


:max_bytes(150000):strip_icc()/Plastic-glow-58e3caf23df78c51623bd3ab.jpg)


Closure
Thus, we hope this article has provided valuable insights into The Science of Luminescence: Unveiling the Secrets of Materials that Glow Under Black Light. We thank you for taking the time to read this article. See you in our next article!