The pH Spectrum of Household Items: Unveiling the Chemistry of Everyday Life
Related Articles: The pH Spectrum of Household Items: Unveiling the Chemistry of Everyday Life
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to The pH Spectrum of Household Items: Unveiling the Chemistry of Everyday Life. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The pH Spectrum of Household Items: Unveiling the Chemistry of Everyday Life

The pH scale, a measure of acidity and alkalinity, plays a crucial role in our daily lives. From the cleaning products we use to the food we consume, understanding the pH of household items can provide valuable insights into their properties and potential effects. This article delves into the diverse pH spectrum of common household items, exploring their chemical nature and highlighting the implications of their acidity or alkalinity.
Understanding the pH Scale
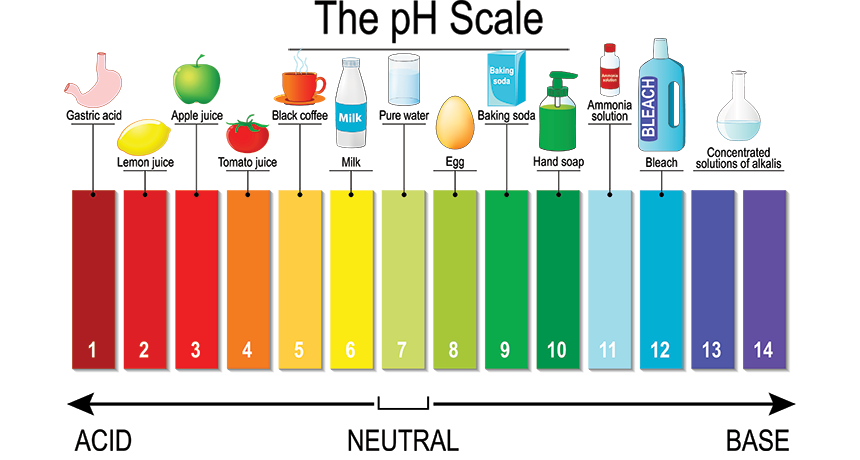
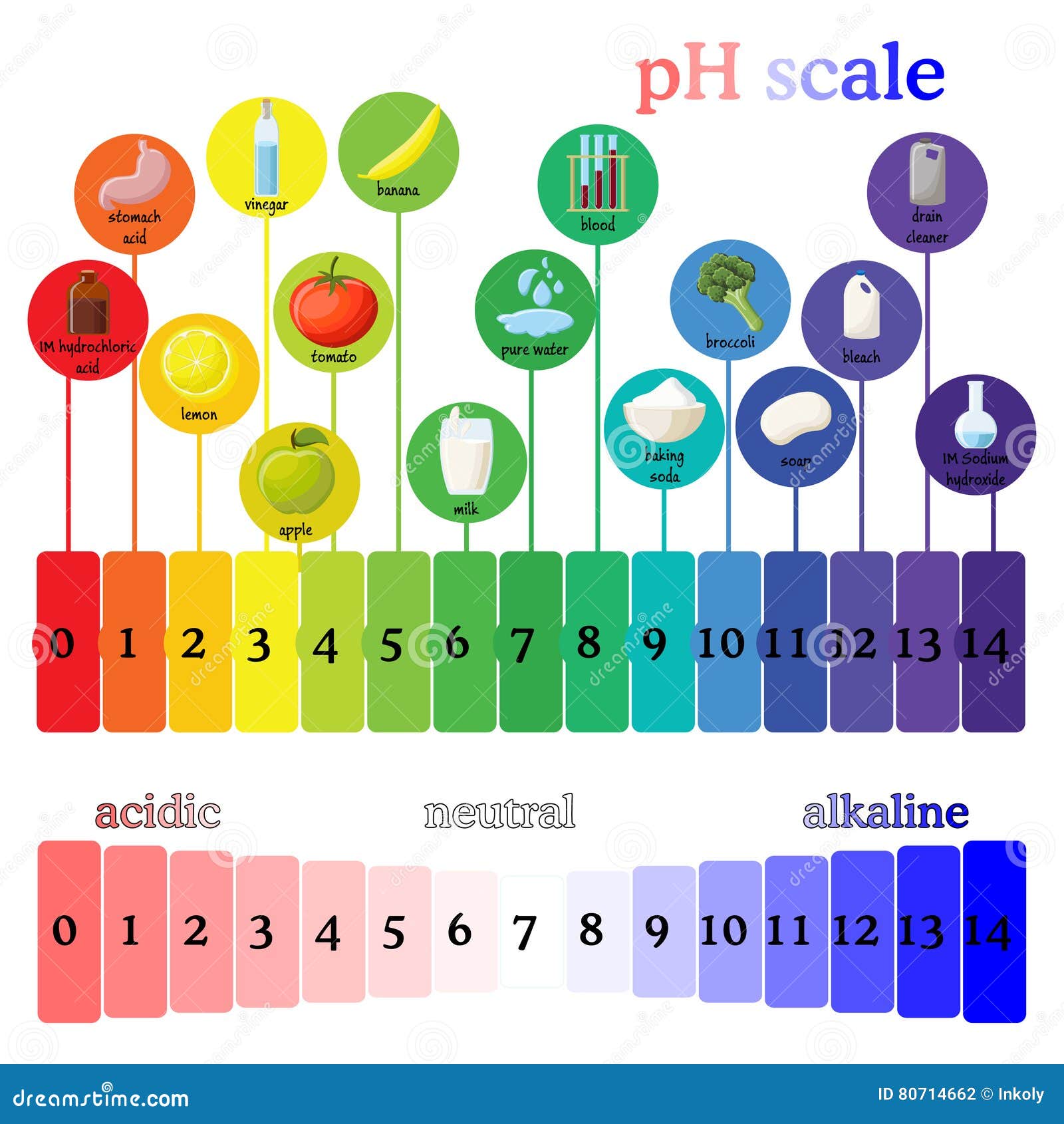
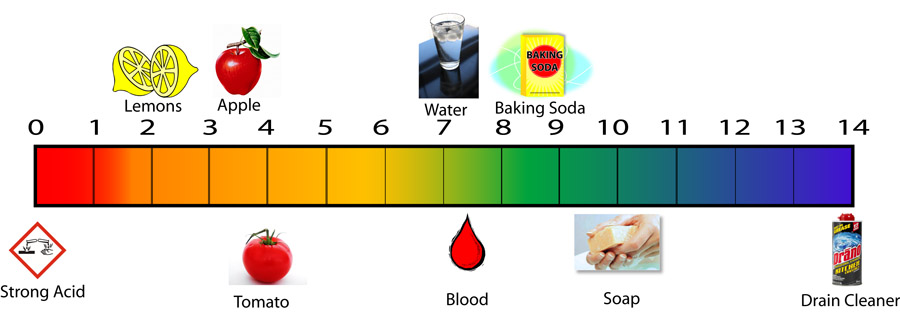
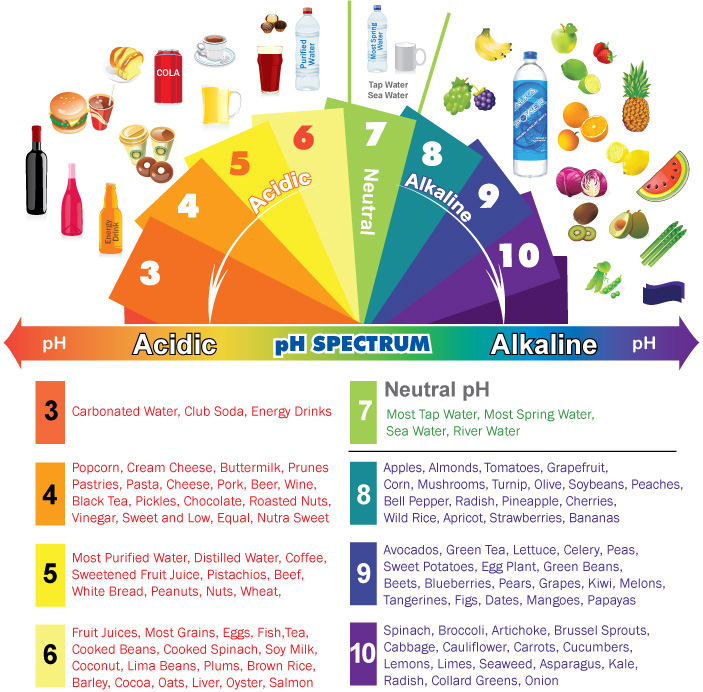
The pH scale ranges from 0 to 14, with 7 representing neutrality. Substances with a pH below 7 are considered acidic, while those with a pH above 7 are alkaline (also known as basic). The lower the pH, the stronger the acid, and the higher the pH, the stronger the base.
Acids in the Household
1. Citrus Fruits: Lemons, limes, oranges, and grapefruits are known for their tangy flavor, which is due to their acidic nature. Their pH typically falls between 2 and 3, making them effective for cleaning and brightening surfaces.
2. Vinegar: This common household staple, made from fermented apple cider or wine, has a pH of around 2.4. Its acidic properties make it a versatile cleaner, effectively removing mineral deposits and grime.
3. Coffee: The brew that fuels many mornings has a pH of approximately 4.5 to 5.5, making it slightly acidic. This acidity can contribute to tooth enamel erosion if consumed in excessive quantities.
4. Tomato Juice: This popular beverage, enjoyed for its rich flavor, has a pH of around 4. Its acidity can contribute to heartburn and acid reflux in some individuals.
5. Baking Soda: While often used as a baking ingredient, baking soda (sodium bicarbonate) is actually a base with a pH of around 8.3. Its alkalinity allows it to neutralize acids, making it useful for cleaning and deodorizing.
6. Soap: Many soaps are slightly alkaline, with a pH ranging from 8 to 10. This alkalinity helps them break down grease and dirt, making them effective cleaning agents.
7. Ammonia: This strong cleaning agent has a pH of around 11.5. Its high alkalinity makes it effective for removing stubborn stains and disinfecting surfaces.
8. Bleach: A powerful disinfectant, bleach has a pH of around 12.6. Its high alkalinity allows it to kill bacteria and viruses, making it a valuable tool for maintaining hygiene.
The Importance of pH Balance
The pH of various household items plays a significant role in their effectiveness and safety. Here are some key considerations:
-
Cleaning: Acids are effective at removing mineral deposits and grime, while bases are better at breaking down grease and dirt. Understanding the pH of cleaning agents allows for more targeted cleaning solutions.
-
Health: The acidity or alkalinity of food and beverages can impact dental health, digestive comfort, and overall well-being. Balancing the pH of our diet is crucial for maintaining good health.
-
Skin and Hair: The pH of skin and hair is naturally slightly acidic. Using products with a similar pH can help maintain healthy skin and hair.
FAQs about pH of Household Items
Q: What is the pH of water?
A: Pure water has a neutral pH of 7.
Q: How can I test the pH of household items?
A: pH test strips, available at most drugstores and online retailers, provide a simple and effective method for determining the pH of various substances.
Q: What are some tips for using household items with different pH levels?
A:
- Dilute strong acids and bases before use. This reduces their potency and minimizes the risk of damage to surfaces or skin.
- Always wear protective gear, such as gloves and eye protection, when handling strong acids and bases.
- Never mix bleach with other cleaning products, especially ammonia. This can create toxic fumes.
- Store acids and bases separately, away from children and pets.
Conclusion
Understanding the pH of household items provides a deeper appreciation for the chemical nature of our everyday lives. From the acidic properties of citrus fruits to the alkalinity of cleaning agents, pH plays a critical role in the effectiveness and safety of various products. By understanding the pH spectrum, we can make informed choices about the products we use and the ways in which we interact with them, ultimately promoting a safer and more informed approach to household management.




Closure
Thus, we hope this article has provided valuable insights into The pH Spectrum of Household Items: Unveiling the Chemistry of Everyday Life. We thank you for taking the time to read this article. See you in our next article!