The pH Spectrum of Everyday Items: A Guide to Understanding Acidity and Alkalinity
Related Articles: The pH Spectrum of Everyday Items: A Guide to Understanding Acidity and Alkalinity
Introduction
With great pleasure, we will explore the intriguing topic related to The pH Spectrum of Everyday Items: A Guide to Understanding Acidity and Alkalinity. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The pH Spectrum of Everyday Items: A Guide to Understanding Acidity and Alkalinity
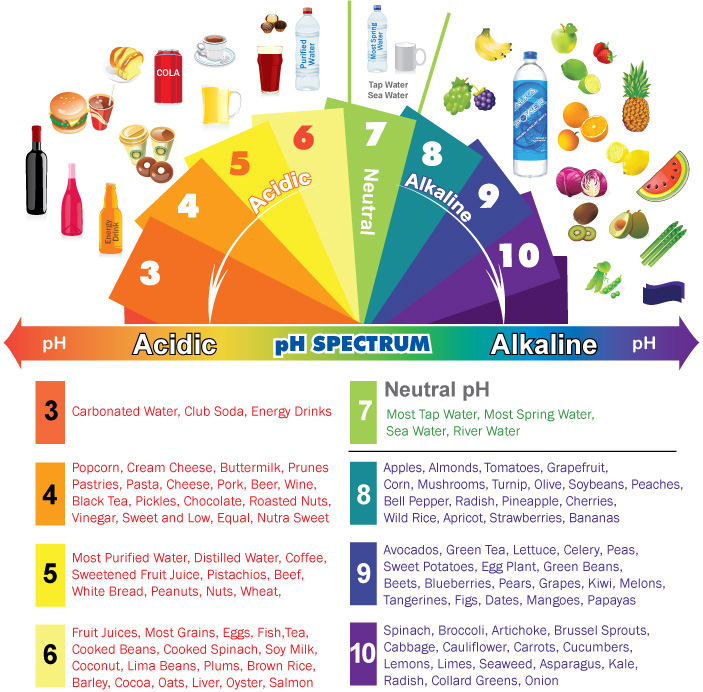
The pH scale, a measure of acidity and alkalinity, plays a crucial role in various aspects of our lives, from the effectiveness of cleaning products to the health of our bodies. Understanding the pH of common household items can provide valuable insights into their properties and how they interact with other substances. This article delves into the pH spectrum of everyday items, highlighting their importance and benefits in a clear and informative manner.
What is pH?
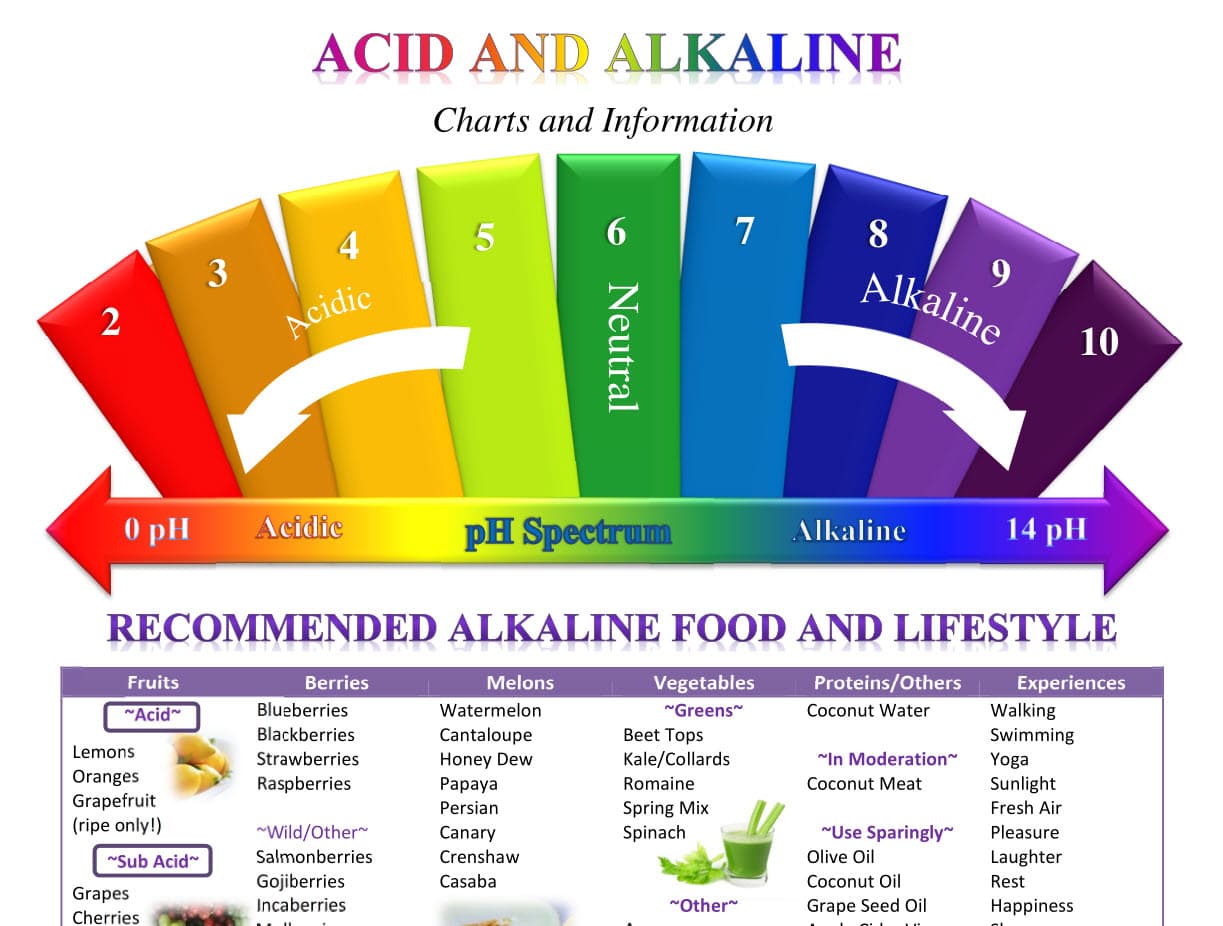
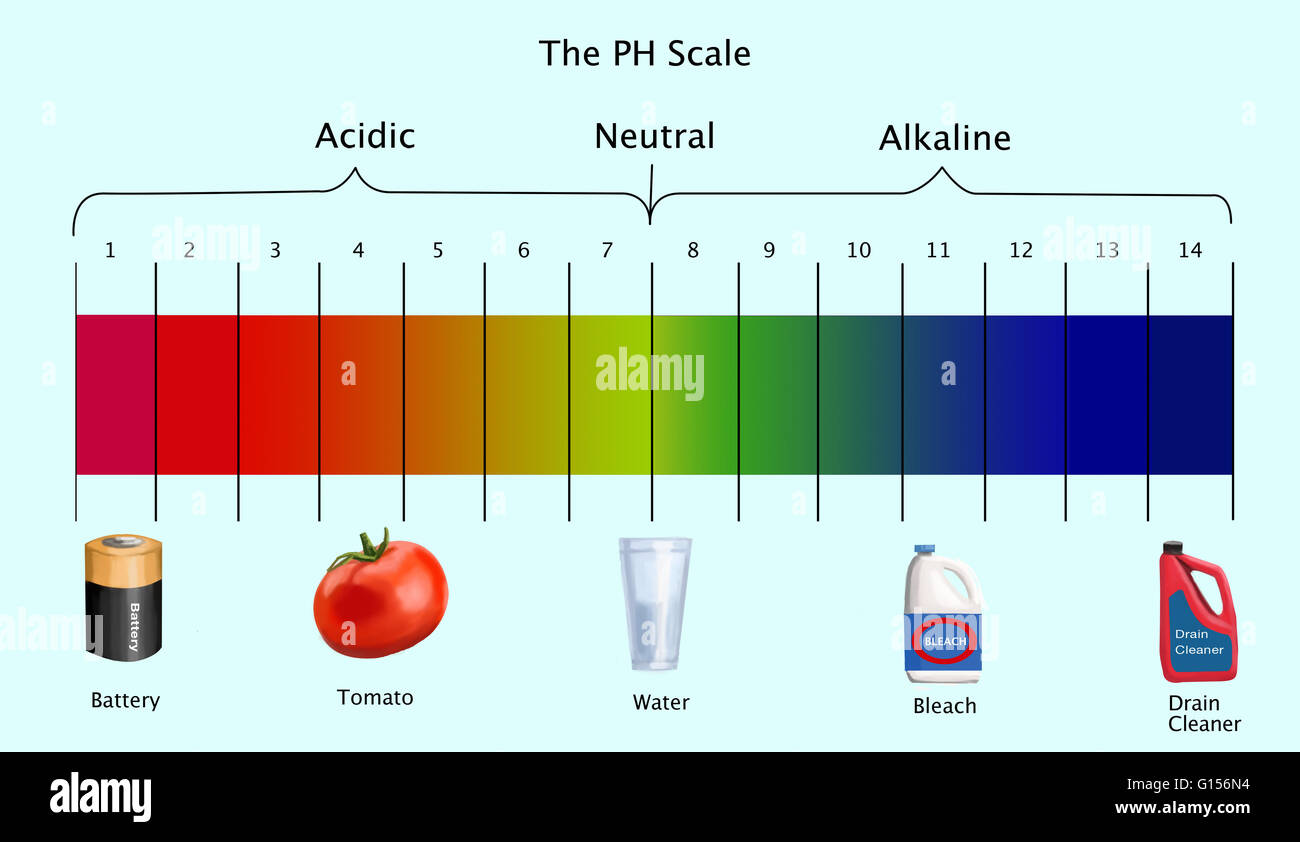
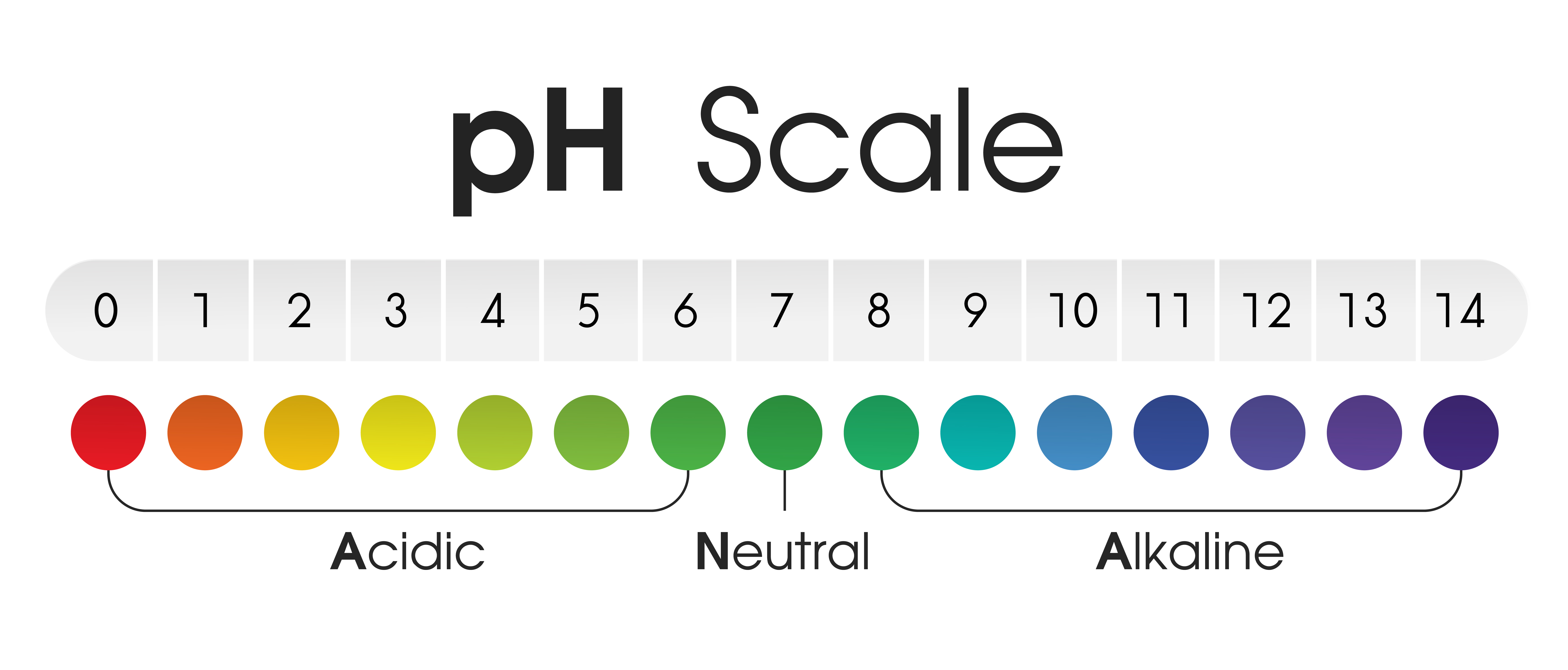
pH stands for "potential of hydrogen" and is a numerical scale ranging from 0 to 14, with 7 representing neutral. Solutions with a pH below 7 are considered acidic, while those with a pH above 7 are alkaline (also known as basic). The scale is logarithmic, meaning that each whole number difference represents a tenfold change in acidity or alkalinity.
The Importance of pH
pH plays a vital role in a wide range of processes, including:
- Chemical Reactions: Many chemical reactions, particularly those involving acids and bases, are highly sensitive to pH.
- Biological Processes: The pH of bodily fluids, such as blood and saliva, is tightly regulated to maintain optimal conditions for physiological processes.
- Environmental Health: The pH of soil and water influences the growth of plants and animals, as well as the effectiveness of wastewater treatment.
- Household Applications: The pH of cleaning products, detergents, and even food influences their effectiveness and safety.
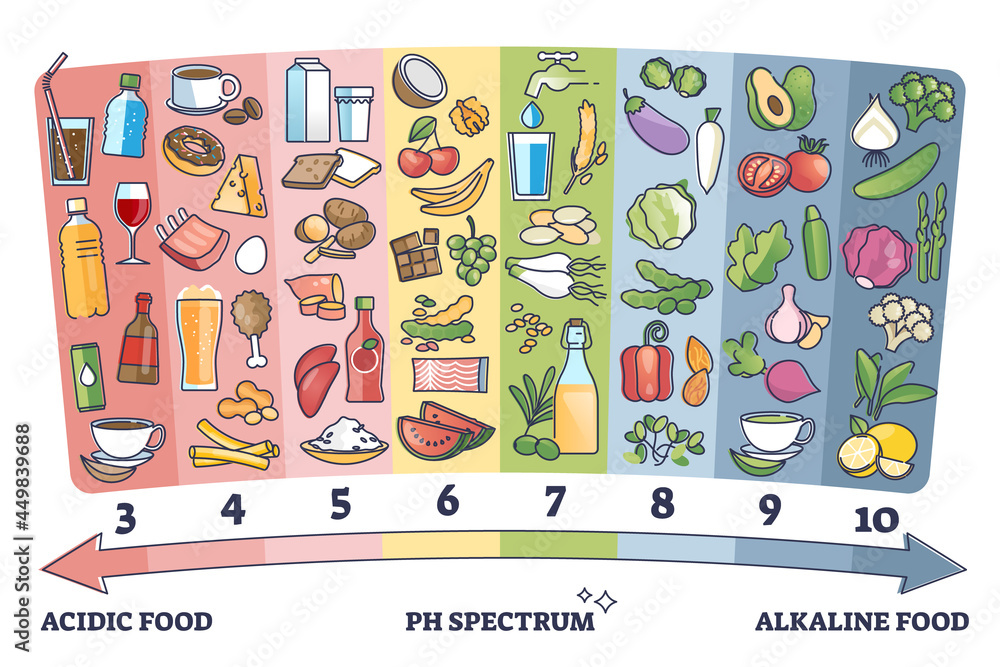
pH of Common Household Items
Here is a breakdown of the pH of common household items, categorized for clarity:
Cleaning Products:
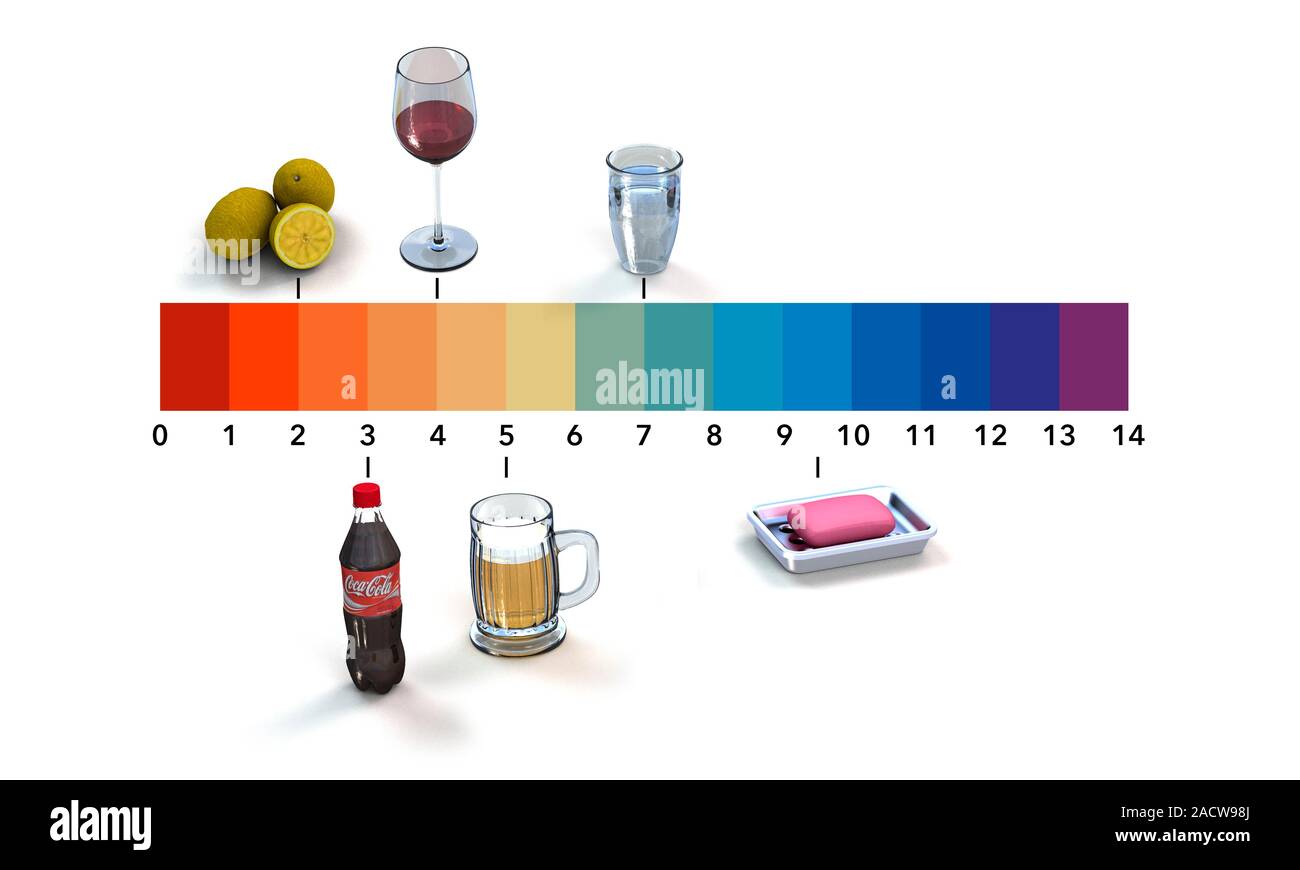
- Vinegar: With a pH of around 2.5, vinegar is a mild acid that is commonly used as a natural cleaning agent. Its acidity helps to break down grease, grime, and mineral deposits.
- Lemon Juice: Similar to vinegar, lemon juice (pH 2.0-2.5) possesses acidic properties that make it effective for cleaning and deodorizing.
- Baking Soda: Baking soda, a common household staple, is alkaline with a pH of around 8.5. Its alkaline nature makes it effective for cleaning grease, removing odors, and neutralizing acidic spills.
- Dish Soap: Dish soaps are typically formulated with a slightly alkaline pH (around 8-9) to effectively cut through grease and food residue.
- Bleach: Bleach is a strong alkaline solution with a pH of around 12.5. Its high alkalinity makes it effective for disinfecting surfaces, but it is important to use bleach with caution due to its corrosive nature.
Food and Beverages:
- Coffee: Coffee is mildly acidic with a pH of around 5.0.
- Tea: The pH of tea varies depending on the type, but generally falls within the slightly acidic range (4.5-5.5).
- Milk: Milk is slightly acidic with a pH of around 6.5.
- Soda: Most sodas are highly acidic, with a pH of around 2.5.
- Vinegar: Vinegar, often used in salad dressings and marinades, is acidic with a pH of around 2.5.
Personal Care Products:
- Shampoo: The pH of shampoo varies depending on the type, but most are formulated with a slightly acidic pH (around 4.5-5.5) to match the natural pH of hair.
- Soap: Soaps are typically alkaline, with a pH of around 9-10.
- Lotion: Lotions are generally formulated with a pH close to neutral (around 6-7) to minimize irritation to the skin.
Other Household Items:
- Water: Pure water has a neutral pH of 7. However, the pH of tap water can vary depending on the source and treatment methods.
- Soil: The pH of soil is crucial for plant growth and can vary widely depending on the composition and location.
- Laundry Detergent: Laundry detergents are typically alkaline, with a pH of around 9-10, to effectively remove dirt and stains.
FAQs
Q: What is the ideal pH for drinking water?
A: The ideal pH for drinking water is considered to be between 6.5 and 8.5. However, the exact optimal pH may vary depending on individual health conditions and the specific water source.
Q: How can I measure the pH of household items?
A: pH can be measured using pH test strips, pH meters, or pH indicator solutions. These tools are readily available online or at most hardware stores.
Q: Can I use vinegar to neutralize a bleach spill?
A: No, mixing vinegar and bleach can create toxic fumes. It is best to use a large amount of water to dilute the bleach and ventilate the area.
Q: Why is it important to maintain a balanced pH in my aquarium?
A: The pH of aquarium water is crucial for the health and well-being of fish and other aquatic life. Maintaining the appropriate pH range ensures optimal conditions for their survival.
Tips
- Use pH-balanced products: When choosing cleaning products, personal care items, and even laundry detergents, consider those that are formulated with a pH that is suitable for their intended use.
- Test the pH of your water: If you are concerned about the pH of your tap water, consider using a pH test kit to measure it.
- Understand the pH of your soil: For gardening purposes, understanding the pH of your soil can help you choose the right plants and fertilizers.
- Be cautious with acidic and alkaline solutions: Always handle acidic and alkaline solutions with caution, as they can cause burns or irritation.
- Consult with a professional: If you have any concerns about the pH of your household items or their potential effects, consult with a professional.
Conclusion
Understanding the pH of everyday items can provide valuable insights into their properties and how they interact with other substances. By being aware of the pH spectrum of household items, individuals can make informed choices about their use, ensuring safety, effectiveness, and a balanced environment. From cleaning products to personal care items, the pH plays a crucial role in various aspects of our daily lives. By incorporating knowledge of pH into our daily routines, we can optimize the effectiveness of products and create a healthier and more balanced environment.



Closure
Thus, we hope this article has provided valuable insights into The pH Spectrum of Everyday Items: A Guide to Understanding Acidity and Alkalinity. We appreciate your attention to our article. See you in our next article!