The pH Scale: Unveiling the Acidity and Alkalinity of Everyday Items
Related Articles: The pH Scale: Unveiling the Acidity and Alkalinity of Everyday Items
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to The pH Scale: Unveiling the Acidity and Alkalinity of Everyday Items. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The pH Scale: Unveiling the Acidity and Alkalinity of Everyday Items
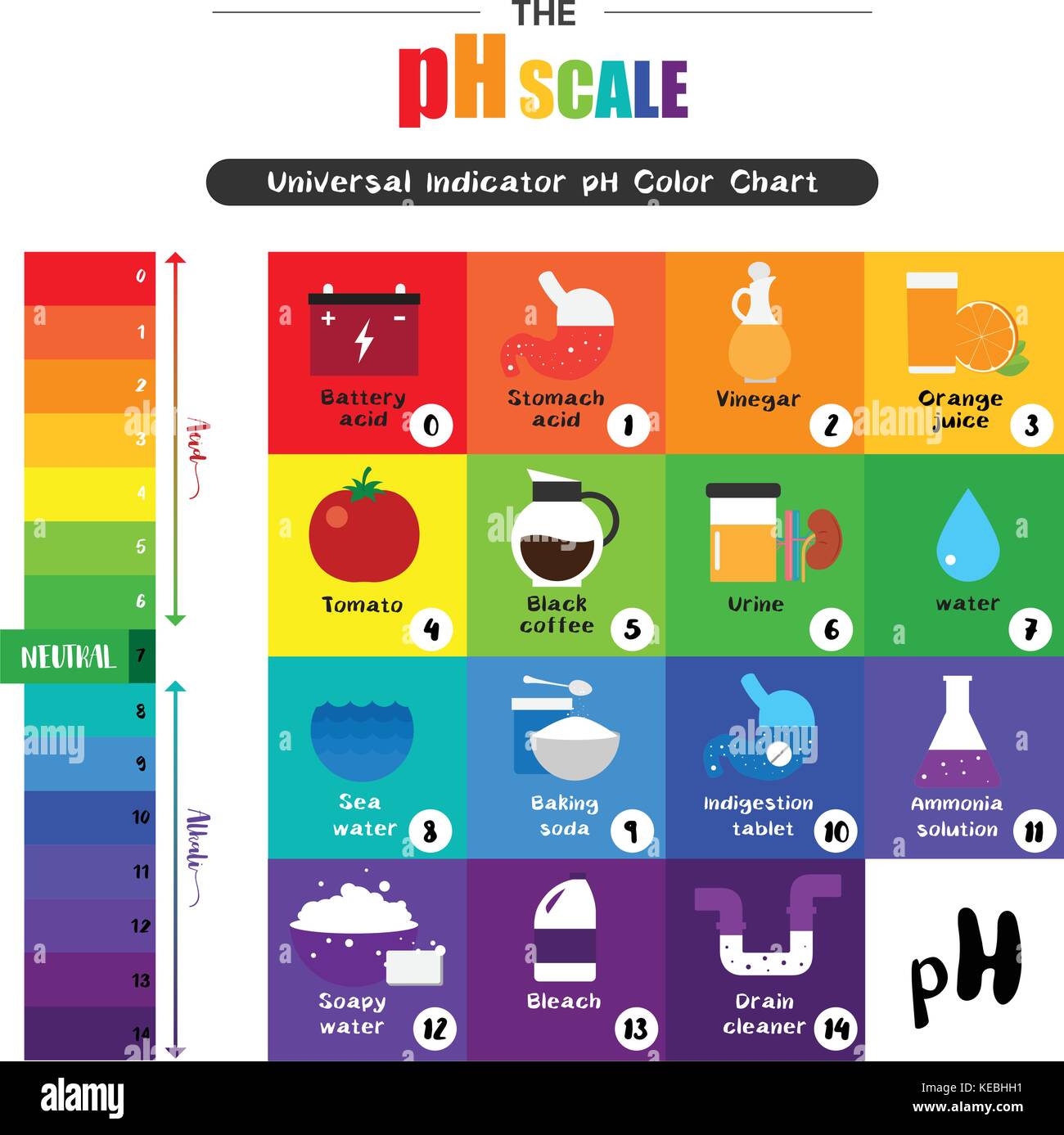
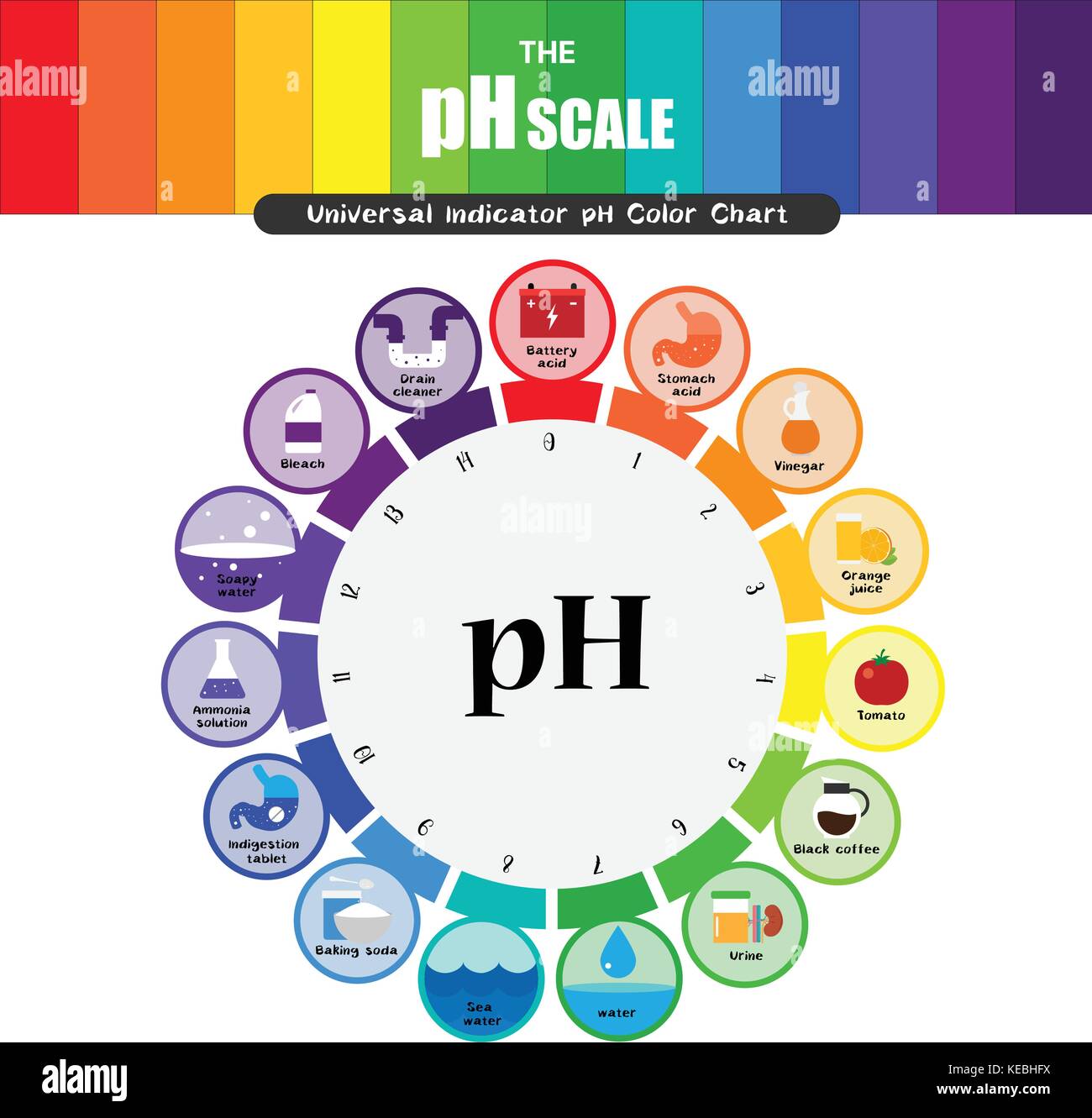
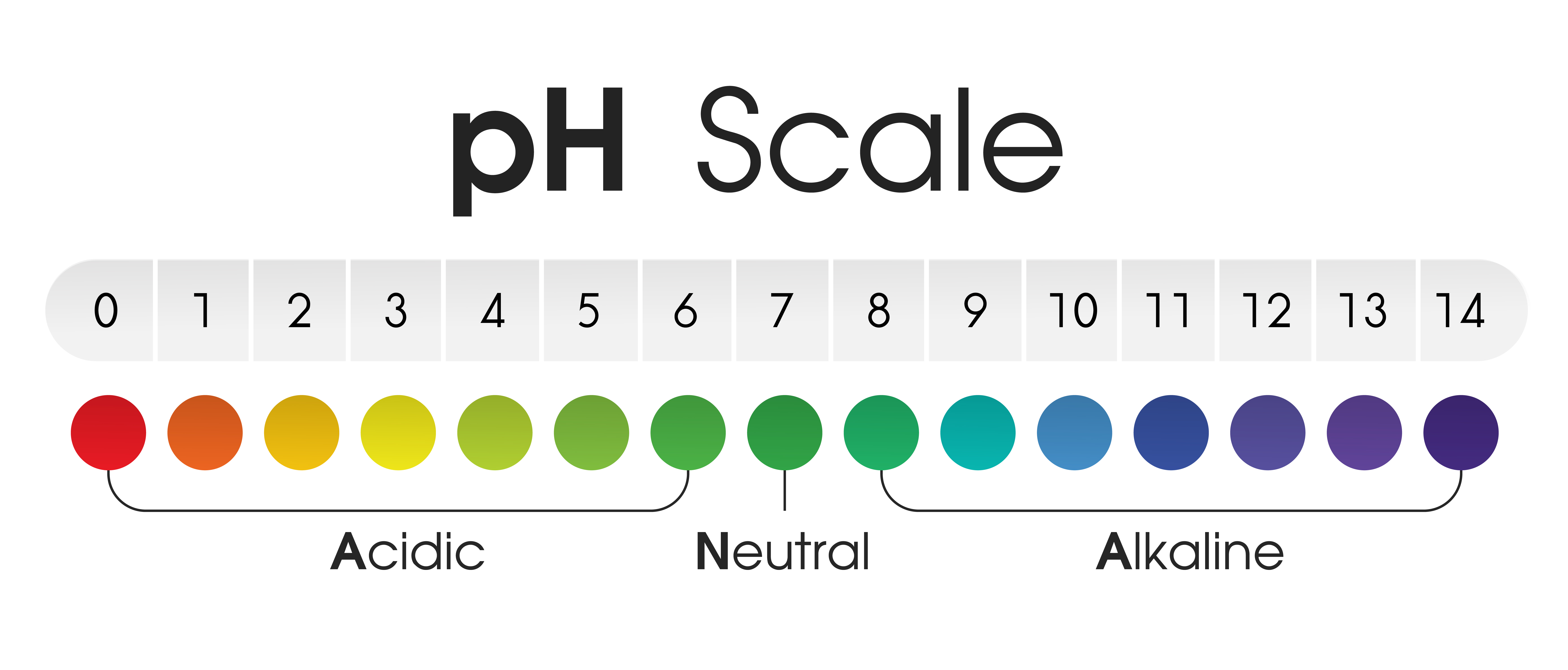
The pH scale, a numerical representation of acidity and alkalinity, plays a crucial role in understanding the chemical nature of various substances, including those found in our homes. This scale, ranging from 0 to 14, with 7 being neutral, provides a standardized method for classifying substances as acidic (pH below 7), alkaline (pH above 7), or neutral. This article delves into the pH levels of common household items, highlighting their significance in various applications and everyday life.
Understanding the pH Scale:
The pH scale is based on the concentration of hydrogen ions (H+) in a solution. A higher concentration of H+ ions indicates a more acidic solution, while a lower concentration indicates a more alkaline solution. The scale is logarithmic, meaning each whole number change in pH represents a tenfold change in the concentration of hydrogen ions.
pH Levels of Common Household Items:
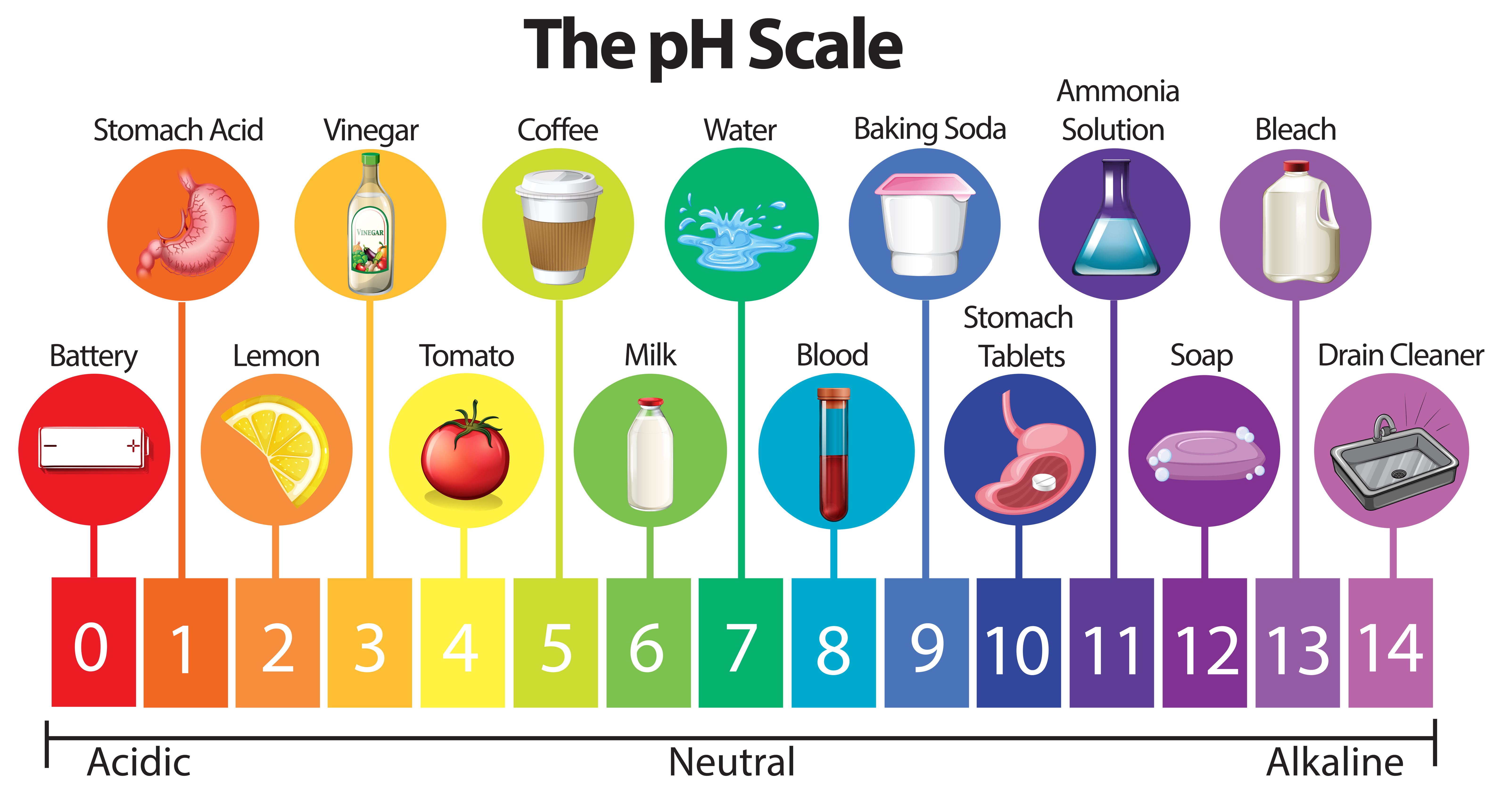
Acids (pH below 7):
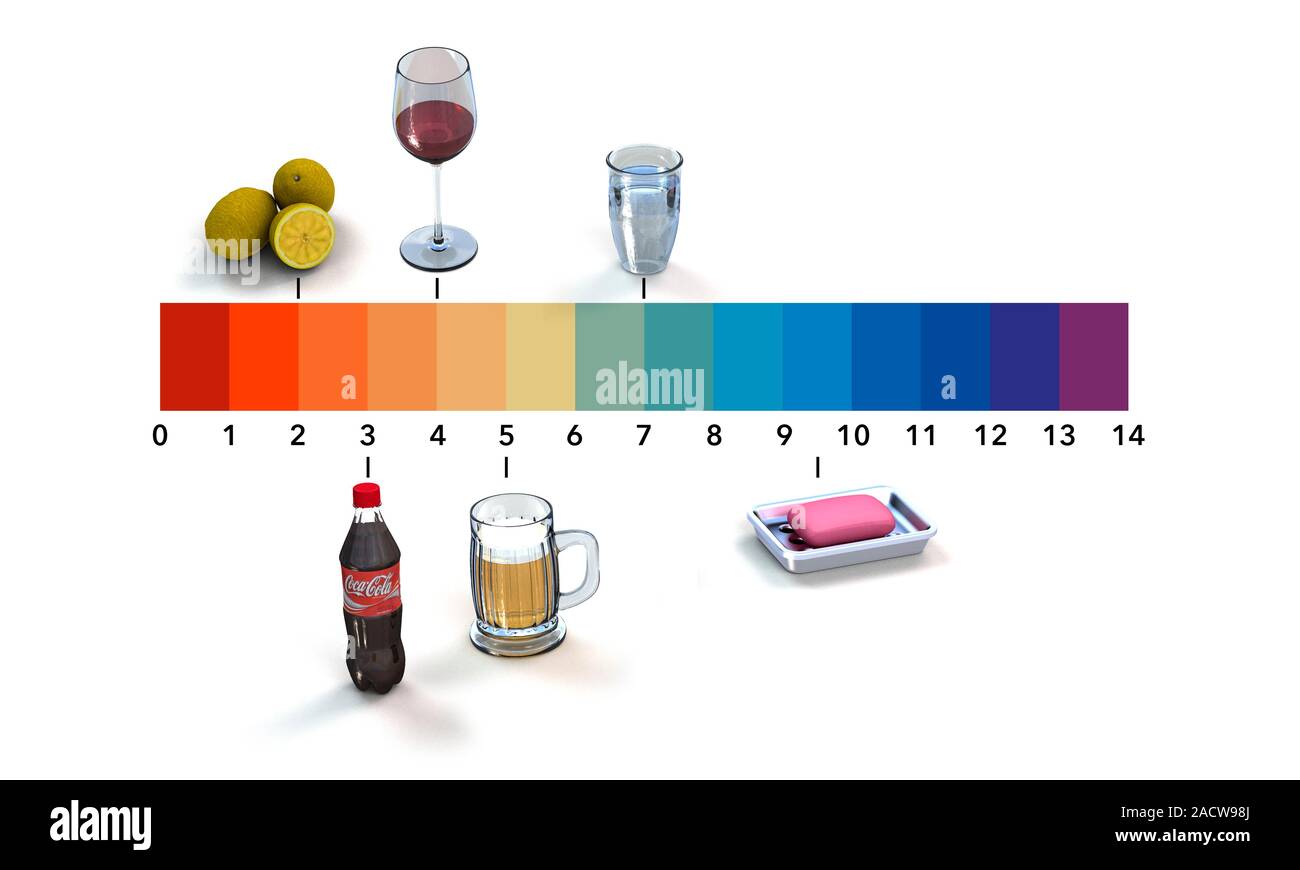
- Vinegar: With a pH ranging from 2 to 3, vinegar is a commonly used household acid. Its acidic nature makes it effective in cleaning, deodorizing, and preserving food.
- Lemon Juice: Similar to vinegar, lemon juice, with a pH of around 2, is a natural acid with a wide range of applications, including cleaning, cooking, and as a natural disinfectant.
- Carbonated Beverages: These beverages, including sodas and sparkling water, typically have a pH between 2 and 4. The high acidity contributes to their characteristic fizz and can also contribute to tooth enamel erosion if consumed excessively.
- Coffee: While the pH of coffee varies depending on the brewing method and type of beans, it generally falls between 4.5 and 5.5. The acidity of coffee can contribute to heartburn in some individuals.
- Tomato Juice: With a pH of around 4, tomato juice is another example of a naturally acidic beverage.
Neutral (pH 7):
- Pure Water: Distilled water, free from dissolved minerals and impurities, has a neutral pH of 7. However, tap water often contains dissolved minerals that can slightly alter its pH.
- Milk: Milk, with a pH of approximately 6.6, is considered slightly acidic but is often classified as neutral.
Alkaline (pH above 7):
- Baking Soda: Baking soda, a common household ingredient, has a pH of around 8.3. Its alkalinity makes it effective in cleaning, deodorizing, and as a natural antacid.
- Ammonia: A powerful cleaning agent, ammonia has a pH of around 11.5. Its strong alkalinity makes it effective in removing grease and dirt but also requires caution due to its corrosive nature.
- Bleach: Bleach, with a pH of around 12.5, is a highly alkaline substance used for disinfecting and whitening. Its strong alkalinity makes it a powerful cleaning agent but also requires careful handling to avoid skin and eye irritation.
- Soap: Most soaps have a pH ranging from 9 to 10. Their alkalinity contributes to their cleaning power by dissolving grease and dirt.
- Dishwashing Detergent: Similar to soap, dishwashing detergent is typically alkaline, with a pH around 10.
Importance of pH Levels in Household Items:
Understanding the pH levels of household items is crucial for several reasons:
- Cleaning Effectiveness: The pH of cleaning products directly influences their effectiveness. Acids are particularly effective at dissolving mineral deposits, while alkalines excel at breaking down grease and oil.
- Safety and Handling: Highly acidic or alkaline substances can be corrosive and require careful handling. Direct contact with skin or eyes can cause irritation or burns.
- Health Considerations: The pH of food and beverages can affect our health. Highly acidic foods can contribute to heartburn, while alkaline foods may help neutralize stomach acid.
- Environmental Impact: The pH of cleaning products can affect the environment. Acidic cleaners can contribute to soil acidification, while alkaline cleaners can disrupt aquatic ecosystems.
FAQs about pH Levels of Household Items:
Q1. What is the pH of human skin?
A1. The pH of human skin is generally slightly acidic, ranging from 4.5 to 6.5. This natural acidity helps protect the skin from harmful bacteria and fungi.
Q2. Can I mix vinegar and bleach?
A2. No, mixing vinegar and bleach is extremely dangerous and should never be done. The reaction creates toxic chlorine gas, which can be fatal.
Q3. How can I neutralize a spilled acidic or alkaline substance?
A3. For acidic spills, use a baking soda solution. For alkaline spills, use a vinegar solution. Always wear protective gear and avoid direct contact with the spilled substance.
Q4. What is the pH of rainwater?
A4. Rainwater is naturally slightly acidic, with a pH of around 5.6, due to the presence of dissolved carbon dioxide. However, pollution can significantly lower the pH, leading to acid rain.
Q5. How can I check the pH of a household item?
A5. You can use pH test strips, which change color depending on the pH level. Alternatively, you can purchase a pH meter for more accurate readings.
Tips for Using Household Items with Different pH Levels:
- Always read the product label: Pay close attention to the manufacturer’s instructions and safety precautions.
- Wear protective gear: When handling strong acids or alkalines, wear gloves, eye protection, and appropriate clothing.
- Dilute concentrated solutions: Always dilute concentrated acids or alkalines before using them.
- Test on a small area first: Before applying a cleaning product to a large surface, test it on a small, inconspicuous area to ensure it does not damage the material.
- Store properly: Keep acids and alkalines separate from each other and in well-ventilated areas.
Conclusion:
The pH scale provides a valuable tool for understanding the chemical nature of household items. By understanding the acidity and alkalinity of these items, we can make informed decisions regarding their use, handling, and potential effects on our health and environment. From cleaning solutions to food and beverages, the pH of these everyday substances plays a significant role in our lives. By being aware of the pH levels and their implications, we can utilize these items safely and effectively while minimizing potential risks.




Closure
Thus, we hope this article has provided valuable insights into The pH Scale: Unveiling the Acidity and Alkalinity of Everyday Items. We hope you find this article informative and beneficial. See you in our next article!