The pH Scale: Understanding the Acidity and Alkalinity of Household Items
Related Articles: The pH Scale: Understanding the Acidity and Alkalinity of Household Items
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to The pH Scale: Understanding the Acidity and Alkalinity of Household Items. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The pH Scale: Understanding the Acidity and Alkalinity of Household Items

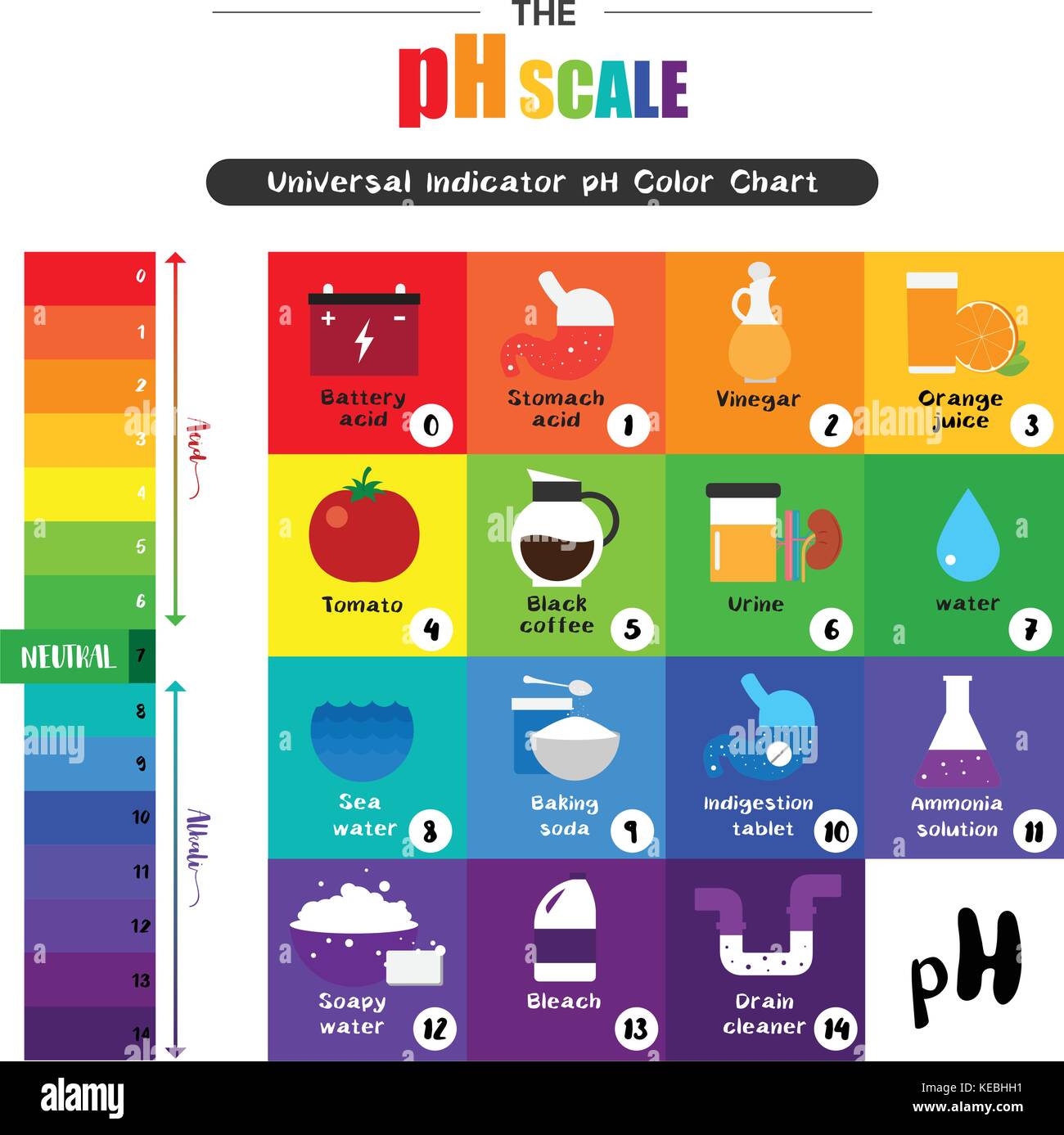
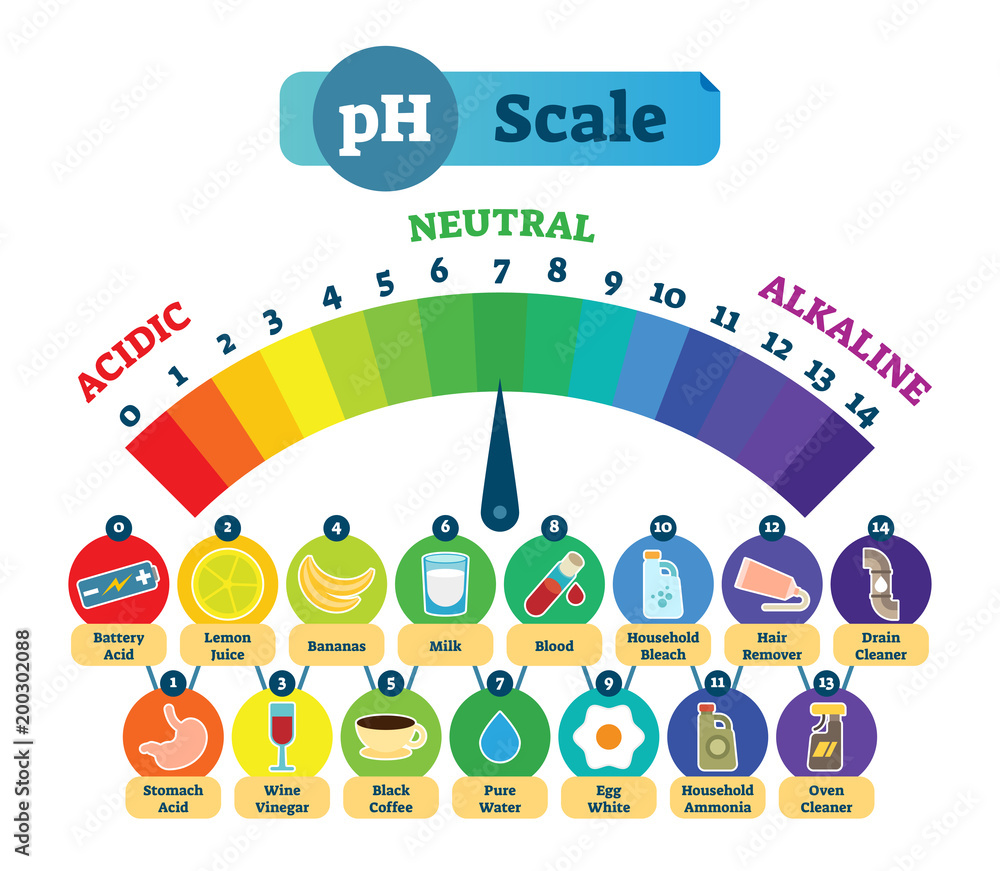
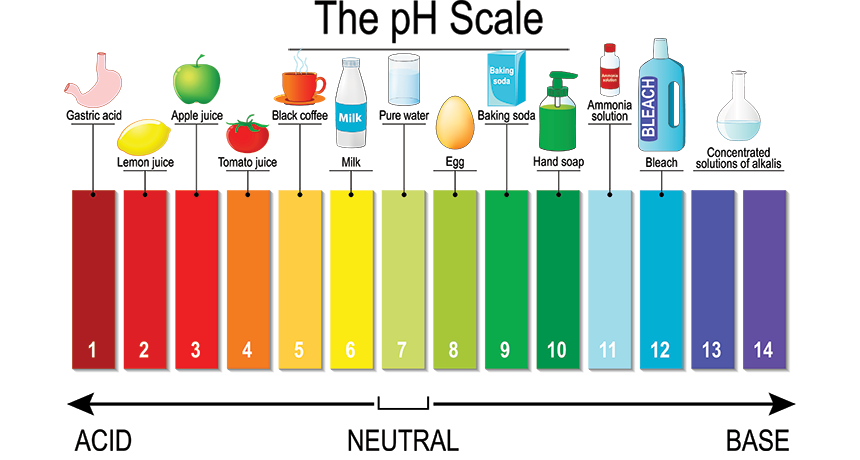
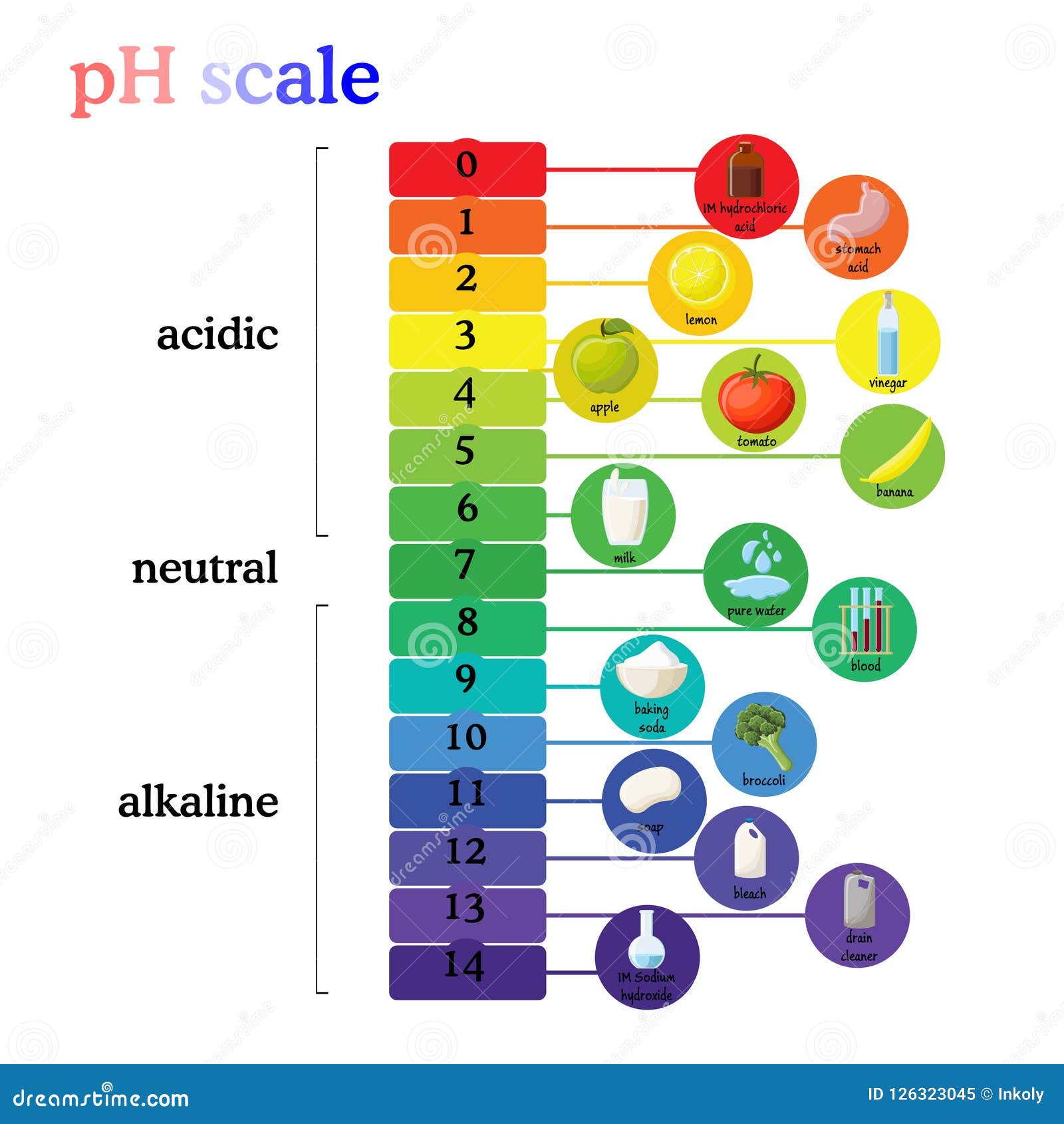
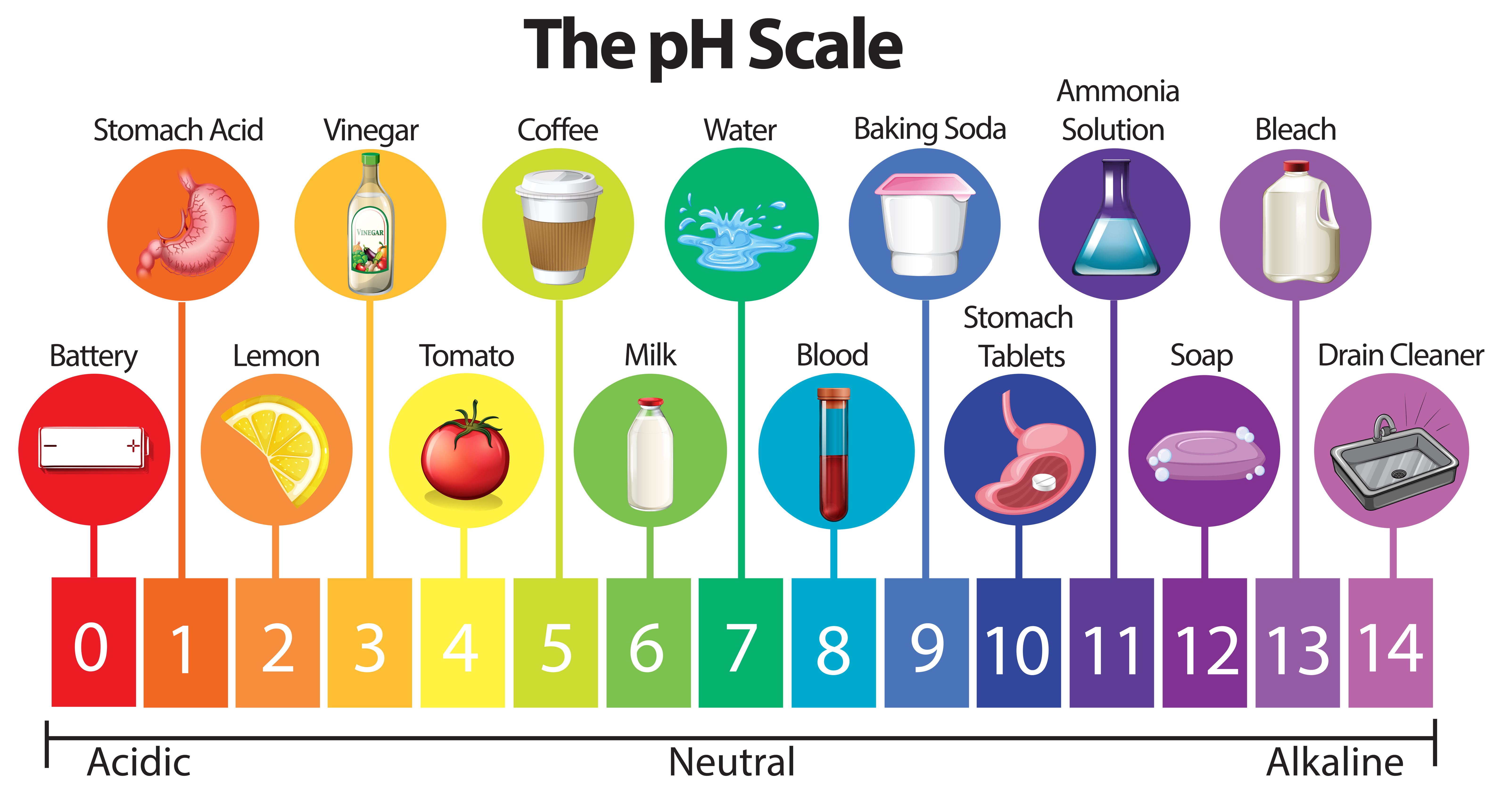
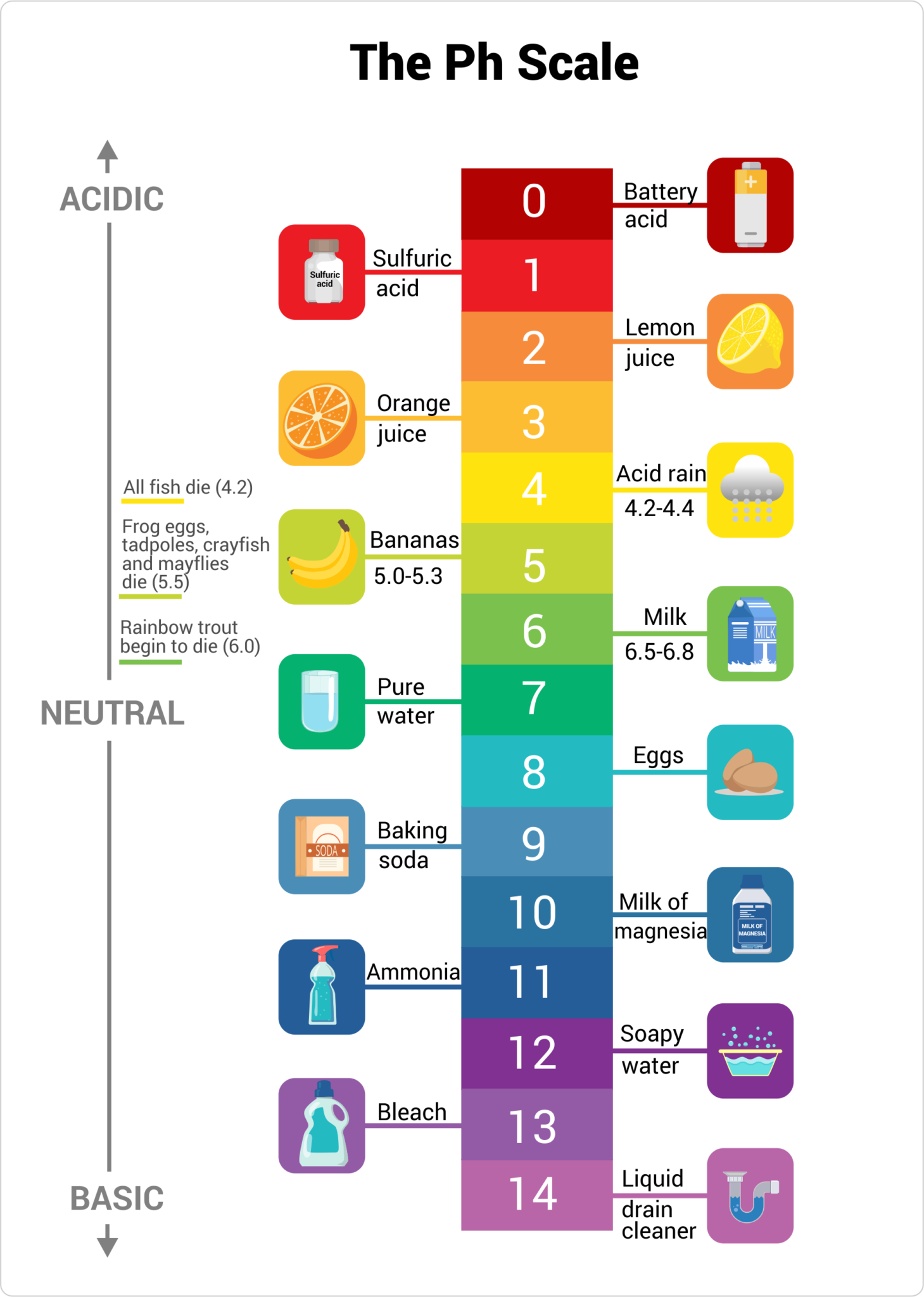
The pH scale, ranging from 0 to 14, quantifies the acidity or alkalinity of a substance. A pH of 7 indicates neutrality, while values below 7 represent acidity and those above 7 indicate alkalinity. Understanding the pH of common household items is crucial for various reasons, including:
- Safety: Knowing the pH of cleaning products helps ensure their safe use, particularly when dealing with delicate surfaces, sensitive skin, or children.
- Effectiveness: Different cleaning tasks require specific pH levels for optimal results. Acidic cleaners excel at removing mineral deposits, while alkaline cleaners are better at dissolving grease and grime.
- Environmental Impact: Certain cleaning products, particularly those with extreme pH values, can negatively impact the environment.
Household Items and their pH Levels:
The following table provides a general overview of the pH levels of common household items. It’s important to note that specific brands and formulations can vary, so always refer to product labels for precise information.
| Item | pH Range |
|---|---|
| Acidic (pH < 7) | |
| Lemon Juice | 2.0 – 2.5 |
| Vinegar | 2.4 – 3.4 |
| Soda | 2.0 – 4.5 |
| Coffee | 4.5 – 5.0 |
| Wine | 2.8 – 3.8 |
| Orange Juice | 3.0 – 4.0 |
| Tomato Juice | 4.0 – 4.5 |
| Neutral (pH = 7) | |
| Pure Water | 7.0 |
| Alkaline (pH > 7) | |
| Baking Soda | 8.3 – 9.0 |
| Ammonia | 11.5 – 12.5 |
| Bleach | 12.5 – 13.5 |
| Liquid Soap | 9.0 – 10.0 |
| Dish Detergent | 7.0 – 11.0 |
| Drain Cleaner | 12.0 – 14.0 |
Understanding the Significance of pH Levels:
- Acidic Solutions: Acidic solutions can dissolve mineral deposits, such as calcium and magnesium, found in hard water. They are often used to clean coffee makers, kettles, and showerheads. However, excessive acidity can damage certain surfaces, like marble or granite.
- Neutral Solutions: Neutral solutions are generally safe for most surfaces and are commonly used for cleaning and rinsing.
- Alkaline Solutions: Alkaline solutions are effective at dissolving grease, oils, and proteins. They are often used for cleaning dishes, laundry, and general household surfaces. However, strong alkaline solutions can be corrosive and should be used with caution.
Examples of pH Levels in Action:
- Vinegar (pH 2.4 – 3.4): Vinegar’s acidity makes it an excellent natural cleaner for removing mineral deposits from hard water stains, cleaning windows, and deodorizing surfaces.
- Baking Soda (pH 8.3 – 9.0): Baking soda’s alkalinity makes it effective for cleaning ovens, scrubbing pots and pans, and deodorizing carpets.
- Bleach (pH 12.5 – 13.5): Bleach’s strong alkalinity makes it a powerful disinfectant, but it can also be corrosive to certain surfaces and should be used with caution.
FAQs about pH Levels of Household Items:
-
Q: How do I measure the pH of a household item?
- A: You can use pH test strips, available at most pharmacies and online retailers. Simply dip the strip into the solution and compare the resulting color to the color chart provided.
-
Q: Can I mix acidic and alkaline solutions together?
- A: Mixing acidic and alkaline solutions can create a chemical reaction that releases heat and potentially harmful fumes. It’s generally best to avoid mixing these solutions unless you are certain of the reaction’s outcome.
-
Q: What happens if I use a cleaning product with the wrong pH?
- A: Using a cleaning product with the wrong pH can damage surfaces, leave streaks or residue, or even be ineffective. Always consult product labels and use them as directed.
Tips for Using pH Levels Effectively:
- Read product labels carefully: Always refer to product instructions and safety warnings before using any cleaning product.
- Test a small area first: Before applying any cleaning product to a large area, test it on a small, inconspicuous spot to ensure it doesn’t damage the surface.
- Use appropriate cleaning tools: Use soft cloths or sponges for delicate surfaces and abrasive scrubbers for tougher cleaning tasks.
- Rinse thoroughly: Always rinse surfaces thoroughly after cleaning with any product, especially those with extreme pH values.
Conclusion:
Understanding the pH of household items is essential for safe and effective cleaning practices. By knowing the acidity or alkalinity of different products, individuals can make informed choices about their cleaning routines, ensuring both safety and effectiveness. By carefully reading product labels, testing products on small areas, and using appropriate cleaning tools, individuals can leverage the power of pH to maintain a clean and healthy home environment.

Closure
Thus, we hope this article has provided valuable insights into The pH Scale: Understanding the Acidity and Alkalinity of Household Items. We appreciate your attention to our article. See you in our next article!