The pH Scale in Everyday Life: Understanding the Chemistry of Our Homes
Related Articles: The pH Scale in Everyday Life: Understanding the Chemistry of Our Homes
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to The pH Scale in Everyday Life: Understanding the Chemistry of Our Homes. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The pH Scale in Everyday Life: Understanding the Chemistry of Our Homes

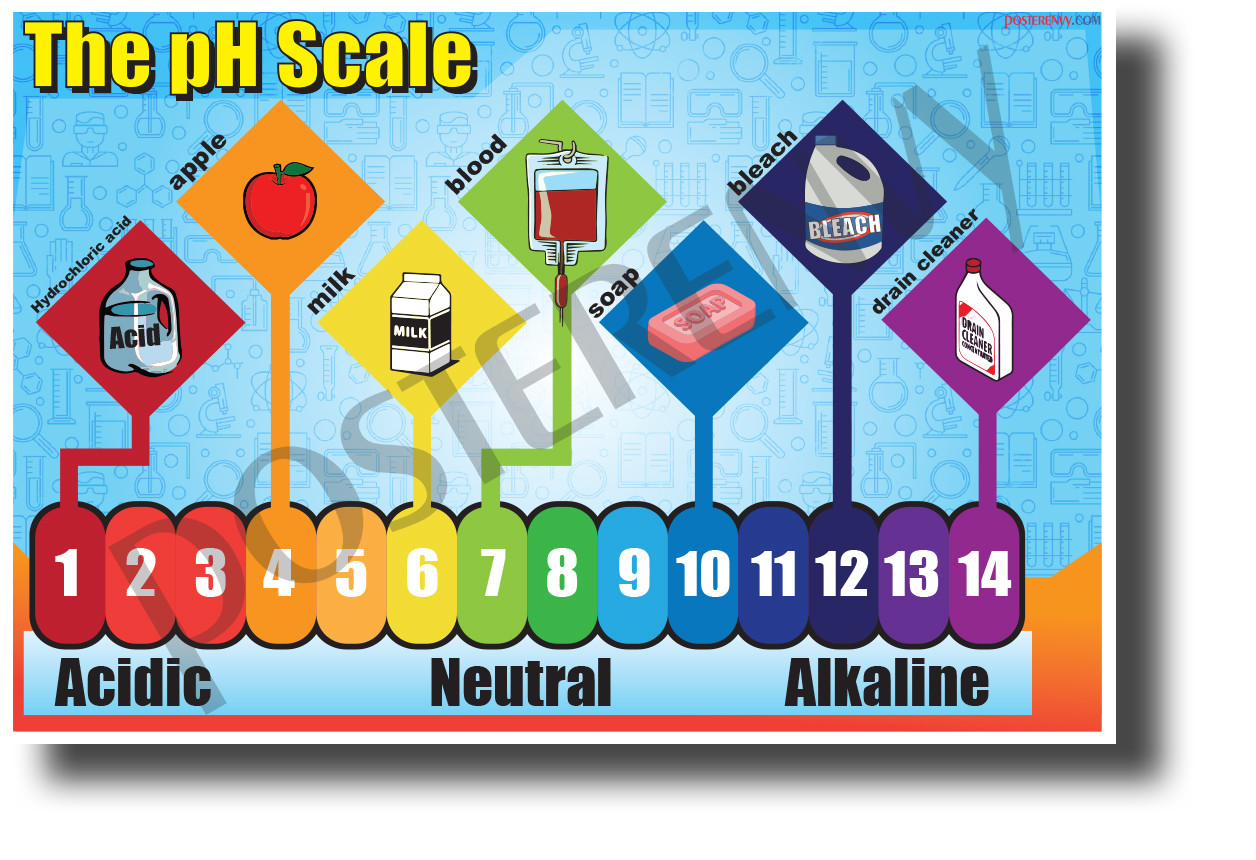
The pH scale, a logarithmic measure of acidity and alkalinity, plays a crucial role in various aspects of our daily lives. From the food we consume to the cleaning products we use, understanding the pH of different household items allows us to make informed decisions regarding their safe and effective use.
Understanding the pH Scale
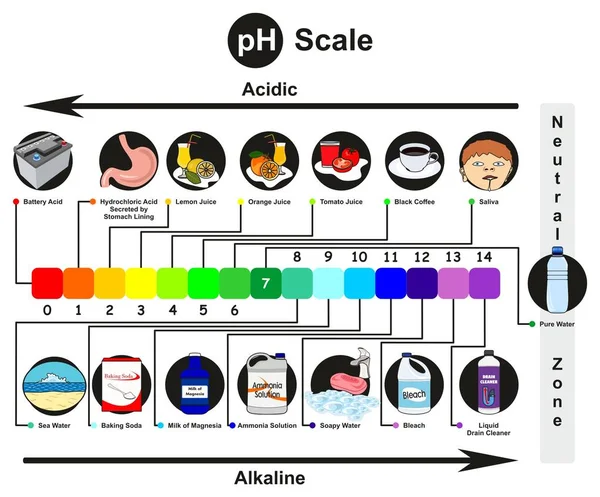
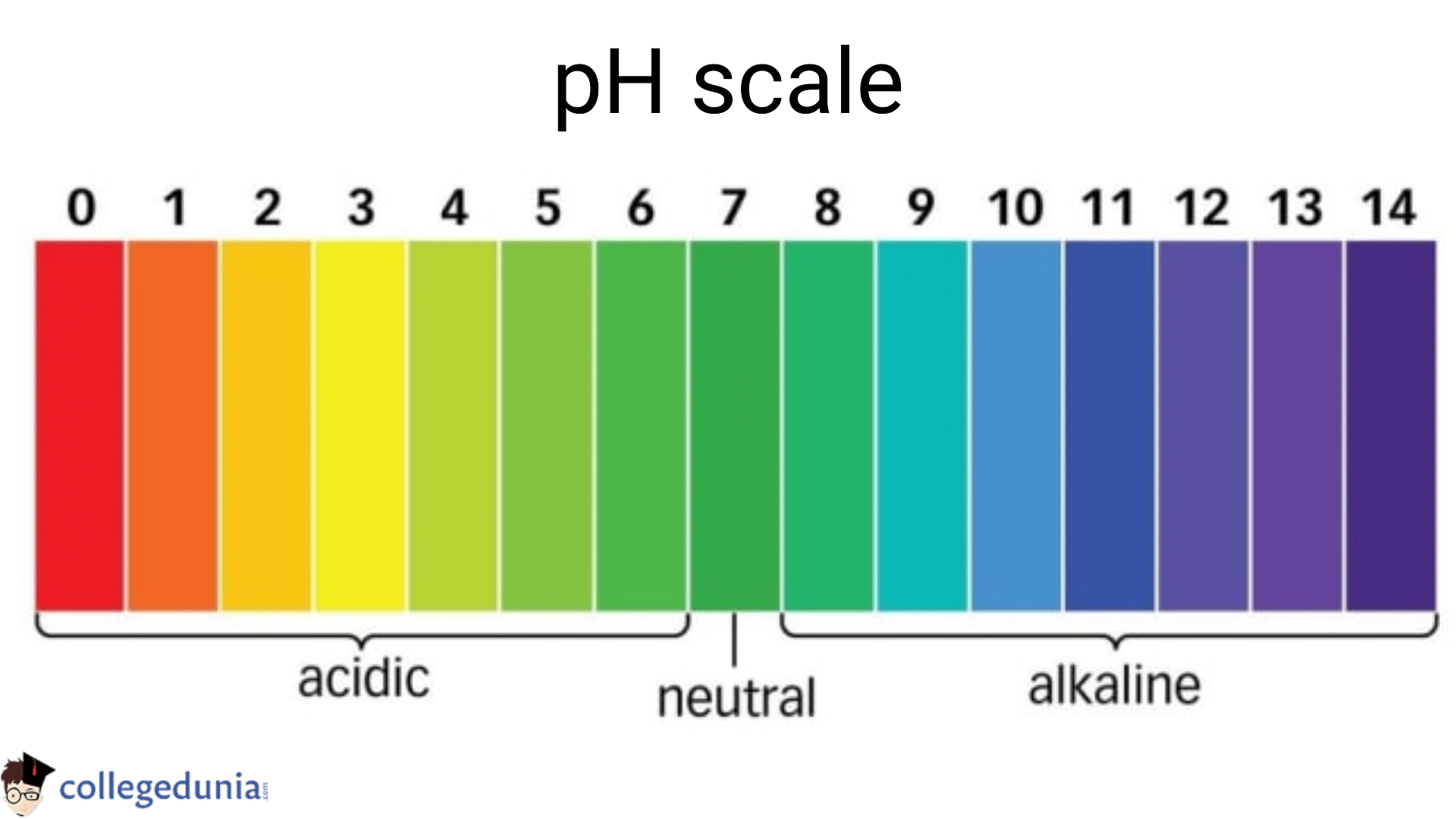
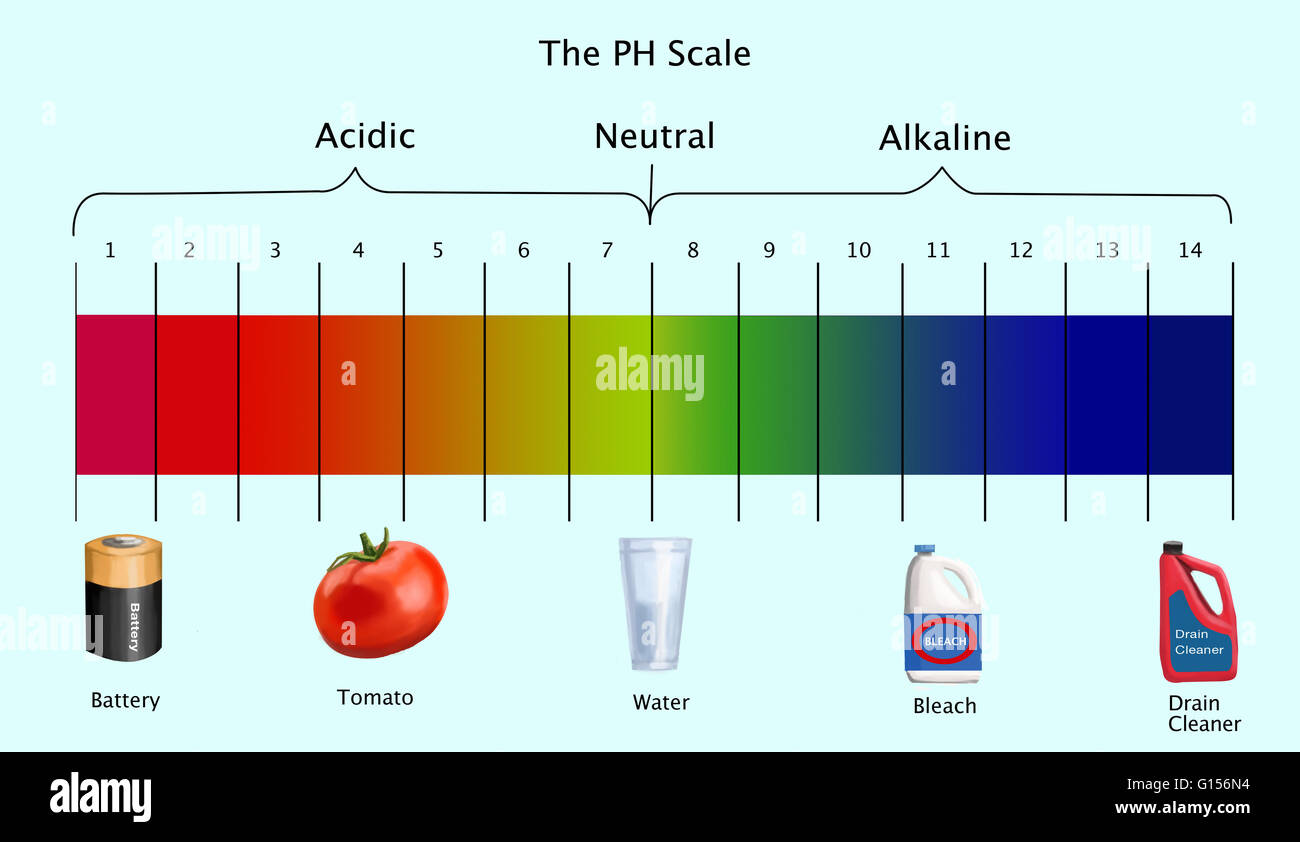
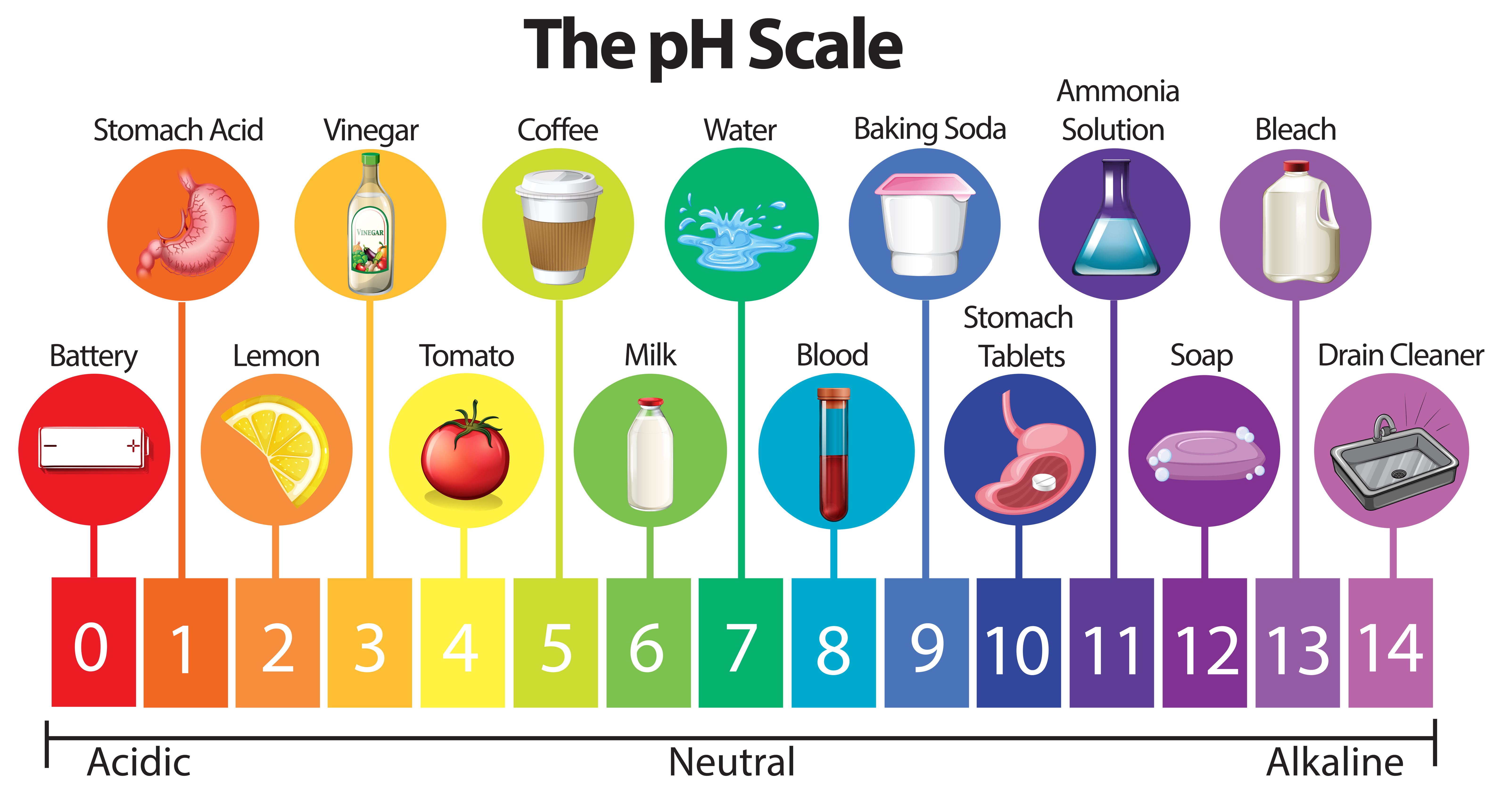
The pH scale ranges from 0 to 14, with 7 representing a neutral solution. Values below 7 indicate acidity, while values above 7 indicate alkalinity. Each whole number on the scale represents a tenfold change in hydrogen ion concentration. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5.
Household Items and Their pH Levels
Here is a breakdown of common household items and their approximate pH levels:
Acidic (pH < 7):
- Vinegar (pH 2-3): A versatile kitchen staple, vinegar’s acidity makes it effective for cleaning, deodorizing, and softening hard water. Its acidic nature also makes it useful for removing mineral deposits and cleaning brass and copper.
- Lemon Juice (pH 2-3): Similar to vinegar, lemon juice’s acidity offers cleaning and deodorizing benefits. It can be used to brighten surfaces, remove stains, and even act as a natural bleach.
- Coffee (pH 4-5): The acidity of coffee contributes to its distinct flavor profile. However, excessive coffee consumption can lead to acid reflux in some individuals.
- Tomato Juice (pH 4-5): Tomatoes, known for their tangy flavor, possess a moderate acidity level. Their juice can be used for cooking and marinating, but its acidity can also be a factor in food spoilage.
- Orange Juice (pH 3-4): Orange juice’s high acidity contributes to its refreshing taste. However, its acidic nature can erode tooth enamel, making it important to consume it in moderation.
- Soft Drinks (pH 2-3): The high acidity of soft drinks contributes to their fizz and sweetness. However, their acidic nature can be detrimental to dental health and contribute to bone loss.
- Battery Acid (pH 0-1): Battery acid is highly corrosive due to its extremely low pH level. It is crucial to handle it with extreme caution and avoid contact with skin and eyes.
Neutral (pH 7):
- Pure Water (pH 7): Although pure water is technically neutral, its pH can vary slightly depending on dissolved minerals and other impurities.
- Milk (pH 6.5-6.8): Milk is considered slightly acidic, but its pH is close to neutral. Its slightly acidic nature contributes to its creamy texture and flavor.
Alkaline (pH > 7):
- Baking Soda (pH 8.3): Baking soda is a common household staple with a slightly alkaline pH. It is widely used as a leavening agent in baking, a natural cleaning agent, and a deodorizer.
- Dish Soap (pH 8-10): Dish soap is formulated with alkaline agents to effectively break down grease and food particles. Its alkalinity can be harsh on delicate skin, so it’s important to use it with caution.
- Bleach (pH 11-13): Bleach is a powerful disinfectant with a highly alkaline pH. It is effective at killing bacteria and viruses, but it can also be harmful to skin, eyes, and respiratory systems.
- Ammonia (pH 11-12): Ammonia is a strong cleaning agent with a highly alkaline pH. It is effective at cleaning surfaces and removing grease, but it can be toxic if inhaled or ingested.
- Lye (pH 13-14): Lye is a highly caustic substance with an extremely alkaline pH. It is used in various industrial processes, but its corrosive nature makes it dangerous to handle.
Importance of pH in Household Items
The pH of household items plays a crucial role in their effectiveness and safety. Understanding the pH of different products allows us to:
- Choose the right cleaning products for specific tasks: Different cleaning tasks require different levels of acidity or alkalinity. For example, acidic cleaners are effective for removing mineral deposits, while alkaline cleaners are better suited for breaking down grease and grime.
- Protect our health and safety: Using cleaning products with extreme pH levels can be harmful to skin, eyes, and respiratory systems. Knowing the pH of cleaning products allows us to use them safely and effectively.
- Maintain the integrity of surfaces: Acidic or alkaline cleaners can damage certain surfaces, such as marble or granite. Understanding the pH of cleaning products allows us to choose the appropriate ones for each surface.
- Optimize cooking and baking: The pH of ingredients affects their taste, texture, and even their ability to rise during baking. Understanding the pH of ingredients allows us to create more flavorful and successful dishes.
FAQs
Q: What are the potential health risks associated with using highly acidic or alkaline cleaning products?
A: Highly acidic or alkaline cleaning products can cause skin irritation, burns, eye damage, and respiratory problems. It is crucial to wear appropriate protective gear, such as gloves and eye protection, when handling these products.
Q: How can I neutralize an acidic or alkaline spill?
A: To neutralize an acidic spill, use a baking soda solution. To neutralize an alkaline spill, use a vinegar solution. In both cases, it is important to wear protective gear and ventilate the area.
Q: How can I determine the pH of a household item?
A: You can purchase pH test strips from most drugstores or online retailers. These strips change color depending on the pH of the solution.
Tips
- Always read product labels carefully: Pay attention to the pH level and any safety warnings.
- Dilute cleaning products as instructed: Diluting strong cleaning products can reduce their harshness and make them safer to use.
- Ventilate the area when using strong cleaning products: This helps to prevent the inhalation of harmful fumes.
- Use a neutralizer if you come into contact with a strong acid or alkali: This can help to minimize the damage to your skin or eyes.
- Store cleaning products safely: Keep them out of reach of children and pets.
Conclusion
The pH scale is an essential tool for understanding the chemistry of our homes. By understanding the pH of different household items, we can make informed decisions regarding their safe and effective use. This knowledge allows us to protect our health, maintain the integrity of our belongings, and enjoy the benefits of a clean and healthy home environment.



Closure
Thus, we hope this article has provided valuable insights into The pH Scale in Everyday Life: Understanding the Chemistry of Our Homes. We thank you for taking the time to read this article. See you in our next article!