The pH of Everyday: Exploring the Acidity and Alkalinity of Household Items
Related Articles: The pH of Everyday: Exploring the Acidity and Alkalinity of Household Items
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to The pH of Everyday: Exploring the Acidity and Alkalinity of Household Items. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The pH of Everyday: Exploring the Acidity and Alkalinity of Household Items

The pH scale, a measure of acidity and alkalinity, is often encountered in scientific contexts, but its influence extends into our daily lives. From cleaning supplies to foodstuffs, the pH of common household items plays a crucial role in their effectiveness and safety. Understanding this invisible factor can enhance our understanding of the products we use and inform our choices for a healthier and more efficient home environment.
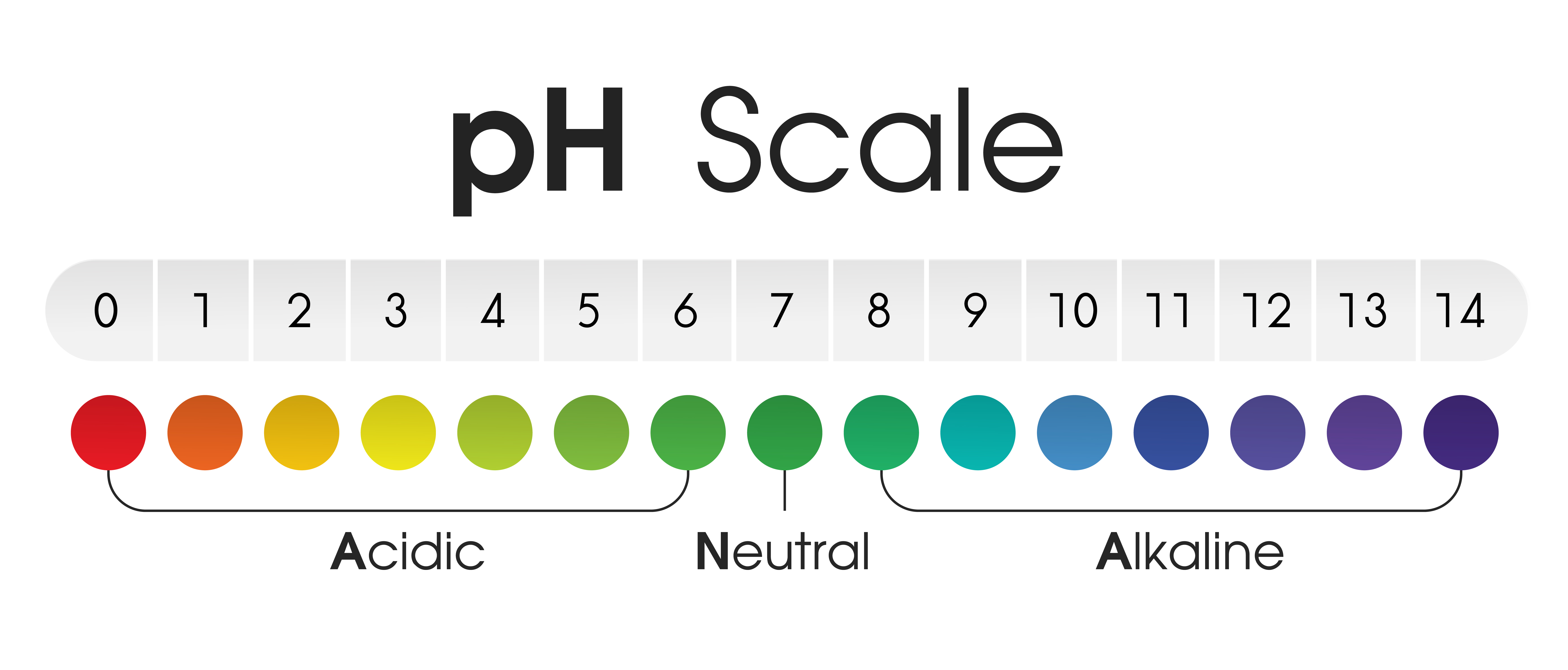
Understanding the pH Scale
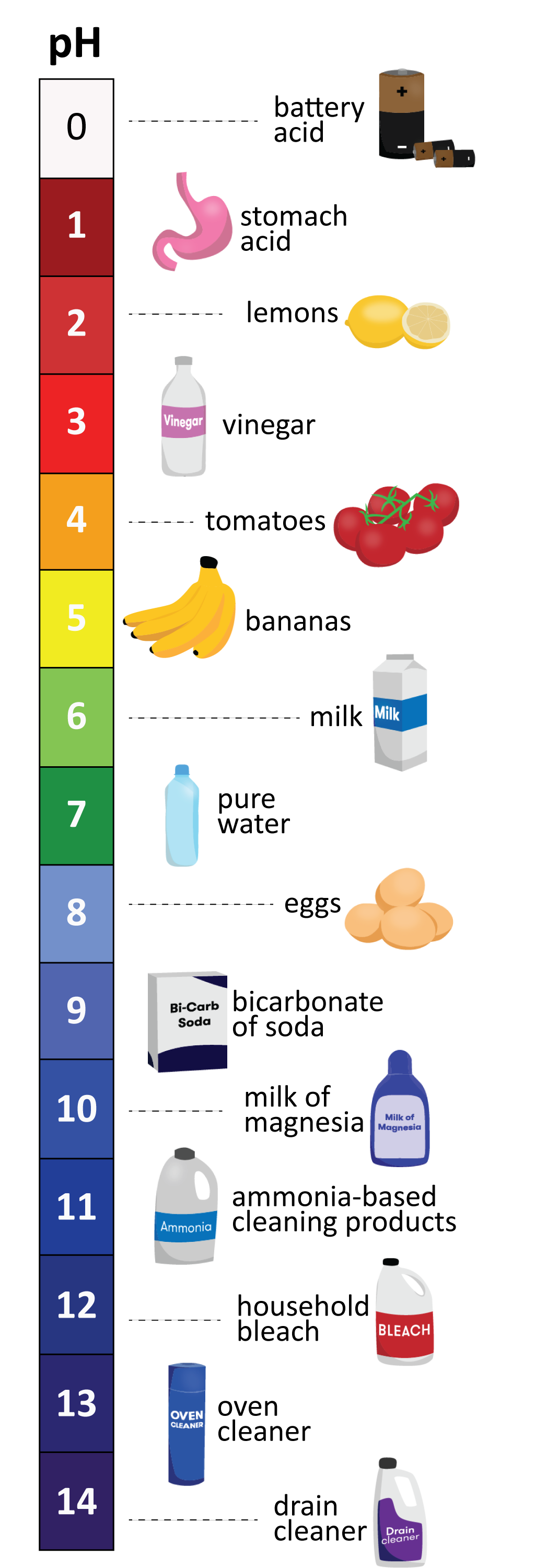
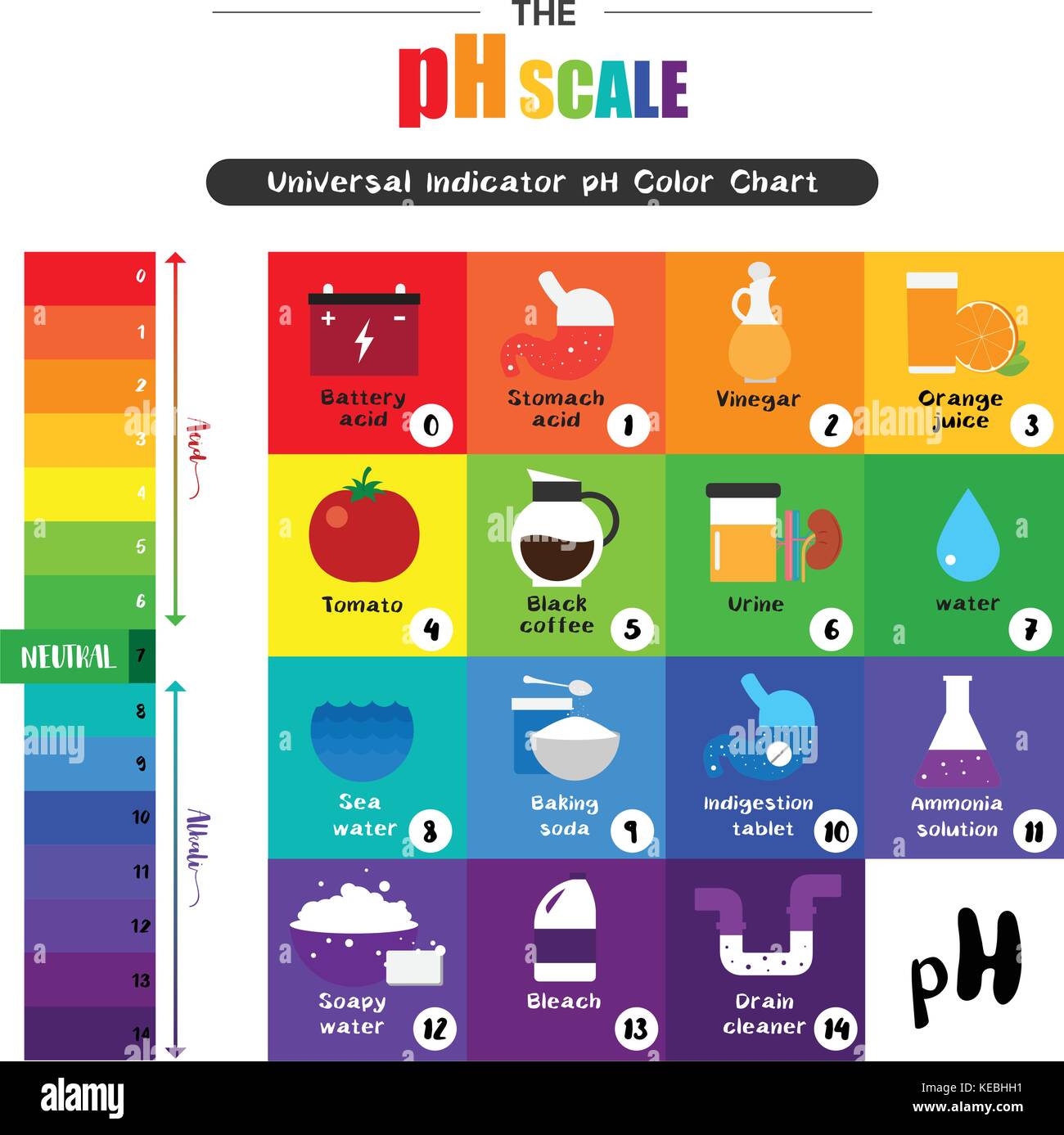
The pH scale ranges from 0 to 14, with 7 being neutral. Values below 7 indicate acidity, increasing in strength as the number decreases. Values above 7 indicate alkalinity, increasing in strength as the number rises.
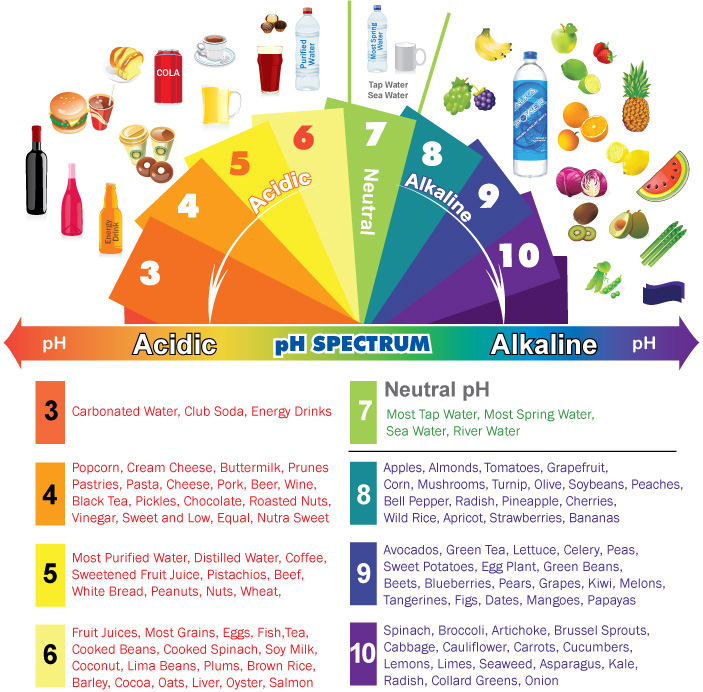
Common Household Items and Their pH
Cleaning Products:
- Vinegar (white): With a pH of around 2.4, vinegar is a mild acid commonly used for cleaning and deodorizing. Its acidity helps break down grease, grime, and mineral deposits.
- Lemon Juice: Similar to vinegar, lemon juice’s pH of 2-3 makes it a natural cleaning agent, effective in removing stains and odors.
- Baking Soda: With a pH of 8.3, baking soda is a mild alkali that acts as a gentle abrasive and deodorizer. It is frequently used for cleaning surfaces, removing stains, and neutralizing odors.
- Dish Soap: The pH of dish soap can vary, but it typically falls between 7 and 10, making it slightly alkaline. This alkalinity helps break down grease and food particles, leaving dishes clean.
- Bleach: Bleach, with a pH of 12.5, is a strong alkaline solution commonly used for disinfecting and whitening. However, its corrosive nature requires careful handling.
Foodstuffs:
- Coffee: With a pH ranging from 4.5 to 5.5, coffee is mildly acidic. Its acidity contributes to its characteristic flavor and aroma.
- Milk: Milk has a pH of around 6.5, making it slightly acidic. This acidity is responsible for its tangy taste and helps preserve its freshness.
- Tomatoes: Tomatoes have a pH of around 4.0, making them quite acidic. This contributes to their bright flavor and tartness.
- Bananas: Bananas have a pH of around 4.5 to 5.5, similar to coffee. Their mild acidity contributes to their sweetness and creamy texture.
- Honey: Honey has a pH of around 3.9, making it acidic. This acidity contributes to its preservative properties and its distinctive flavor.
Personal Care Products:
- Shampoo: Shampoo pH can vary depending on hair type and purpose. Most shampoos fall between 4.5 and 6.5, making them slightly acidic. This helps to maintain the hair’s natural pH balance.
- Soap: Soap, typically alkaline, has a pH range of 9 to 11. This alkalinity helps to remove dirt and oil from the skin.
- Toothpaste: Toothpaste is usually slightly acidic, with a pH ranging from 4.5 to 5.5. This helps to neutralize plaque and prevent cavities.
The Importance of pH in Daily Life
- Cleaning: The pH of cleaning products influences their effectiveness in removing dirt, grease, and stains. Understanding the pH of different cleaning agents allows for targeted cleaning solutions, maximizing their efficiency and minimizing potential damage.
- Food Preservation: The pH of food influences its shelf life. Acidic foods, like pickles and jams, are naturally preserved due to their lower pH, which inhibits bacterial growth.
- Personal Care: The pH of personal care products plays a role in maintaining the skin and hair’s natural pH balance. Using products with a pH close to the skin’s natural pH can help prevent irritation and maintain healthy skin and hair.
- Environmental Impact: The pH of wastewater can affect the environment. Industrial and agricultural runoff can alter the pH of water bodies, impacting aquatic life and the ecosystem.
FAQs about pH of Common Household Items
1. What is the pH of water?
The pH of pure water is 7, making it neutral. However, tap water can vary in pH due to dissolved minerals and other factors.
2. Why is it important to know the pH of cleaning products?
Knowing the pH of cleaning products helps in selecting the right product for the task at hand. For example, a highly acidic product might be effective for removing mineral deposits but could damage delicate surfaces.
3. How can I check the pH of a household item?
You can use pH test strips or a digital pH meter to determine the pH of household items.
4. Is it safe to mix cleaning products with different pH levels?
Mixing cleaning products with different pH levels can be dangerous, as it can release harmful fumes. It is generally recommended to avoid mixing cleaning products unless specifically instructed by the manufacturer.
5. What are the health risks associated with using products with extreme pH values?
Products with extremely high or low pH values can cause skin irritation, burns, and other health problems. It is important to handle such products with caution and follow safety instructions provided by the manufacturer.
Tips for Using Household Items with Different pH Values
- Always read the product label: Manufacturers provide instructions on the safe use and handling of their products.
- Wear gloves and protective clothing: When handling cleaning products with extreme pH values, it is advisable to wear gloves and protective clothing to prevent skin contact.
- Dilute strong cleaning agents: Diluting strong cleaning agents with water can reduce their pH and make them safer to use.
- Test on a hidden area first: Before using a new cleaning product, test it on a hidden area of the surface to ensure it does not cause damage.
- Ventilate the area: When using cleaning products, ensure adequate ventilation to prevent the accumulation of fumes.
Conclusion
The pH of common household items plays a significant role in their effectiveness, safety, and impact on the environment. Understanding this invisible factor can empower individuals to make informed decisions about the products they use, promoting a healthier and more sustainable home environment. By carefully considering the pH of everyday items, we can leverage their properties for cleaning, preservation, and personal care, while minimizing potential risks and environmental impact.




Closure
Thus, we hope this article has provided valuable insights into The pH of Everyday: Exploring the Acidity and Alkalinity of Household Items. We appreciate your attention to our article. See you in our next article!