The Hidden Language of Household Substances: Understanding pH
Related Articles: The Hidden Language of Household Substances: Understanding pH
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to The Hidden Language of Household Substances: Understanding pH. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The Hidden Language of Household Substances: Understanding pH

The world around us is teeming with chemicals, many of which we encounter daily in our homes. These substances, from the cleaning products we use to the food we consume, possess a crucial property known as pH, which dictates their acidity or alkalinity. Understanding pH levels is essential for navigating the chemical landscape of our homes safely and effectively.
Delving into the pH Scale: A Spectrum of Acidity and Alkalinity
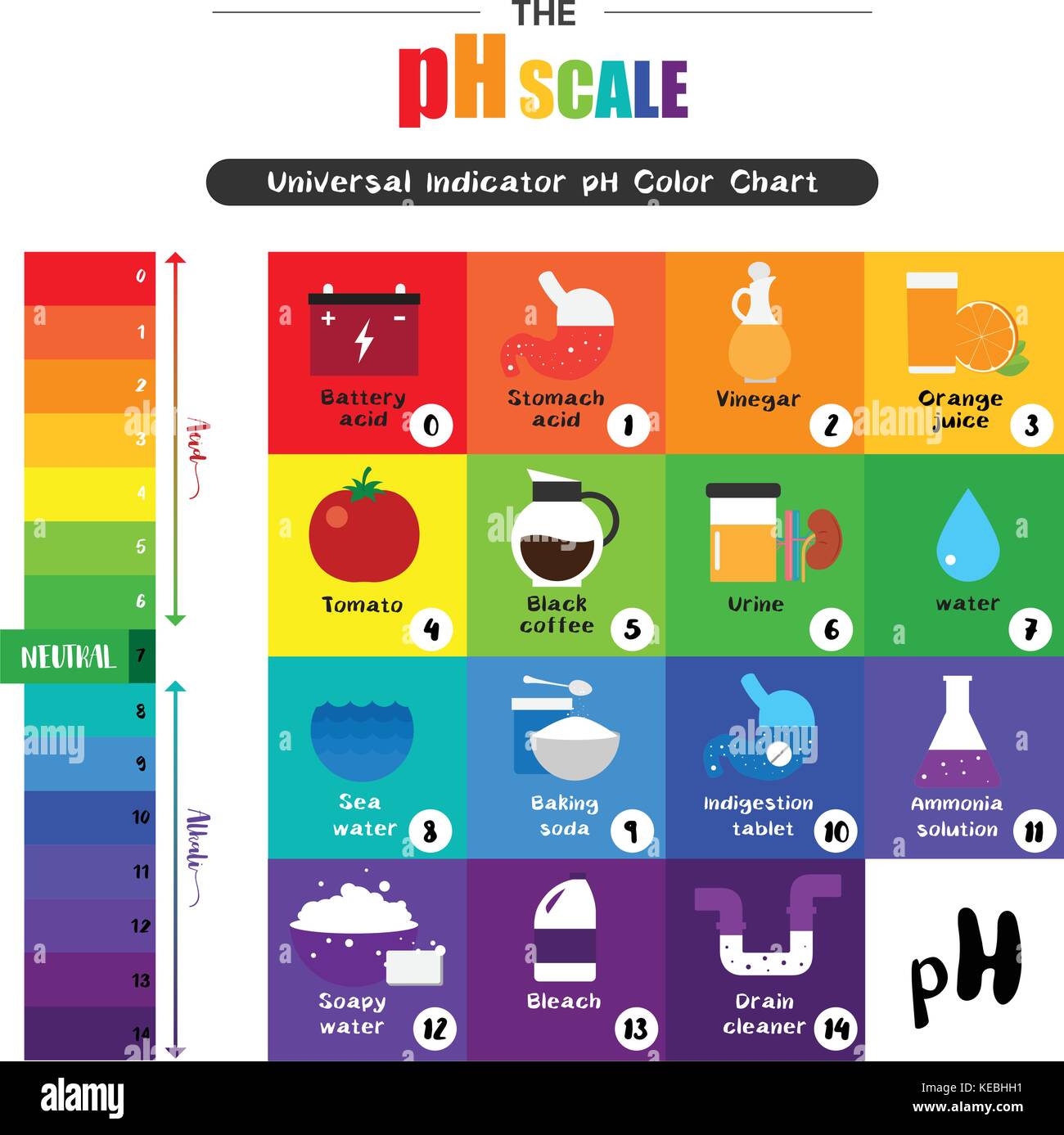
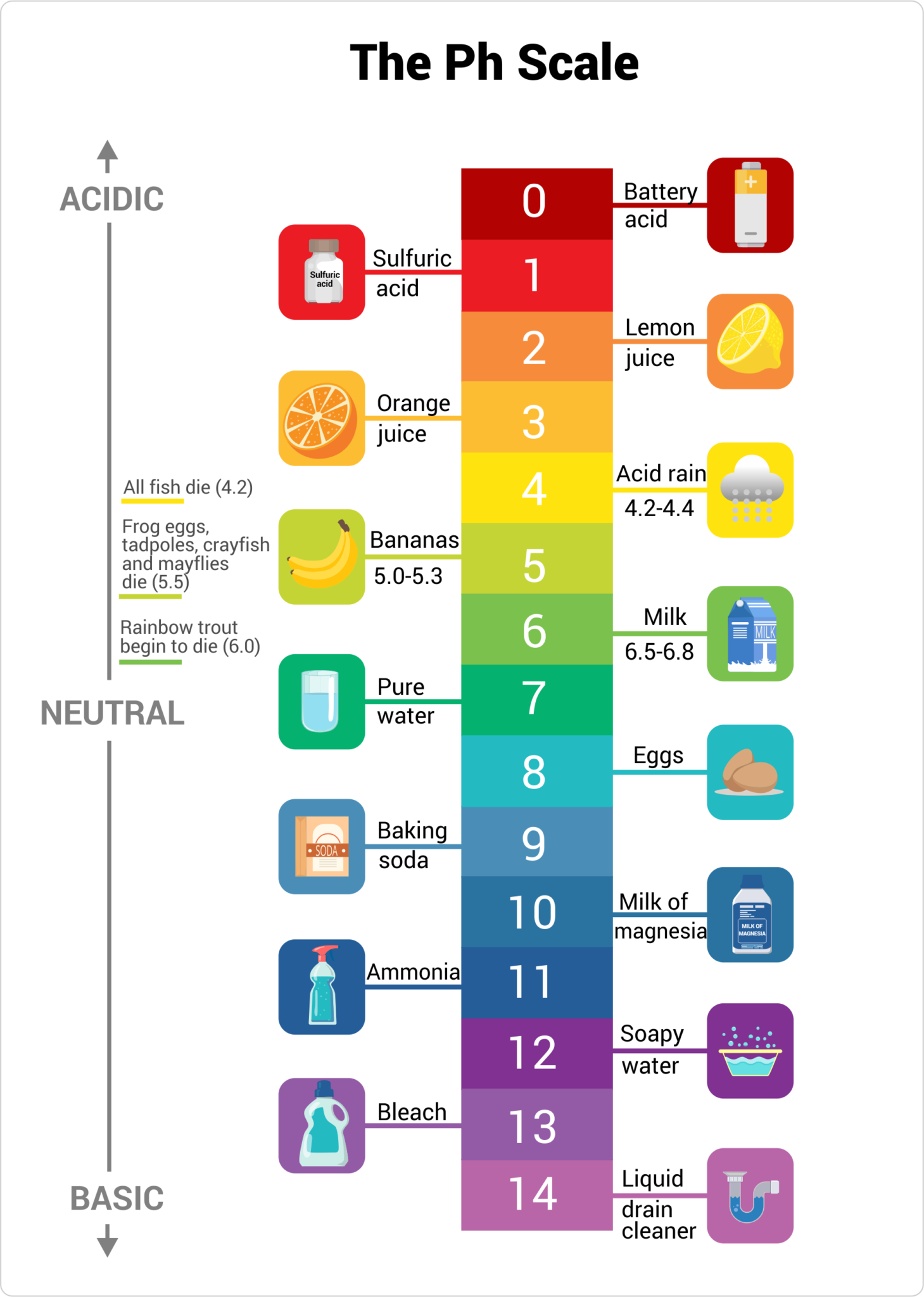
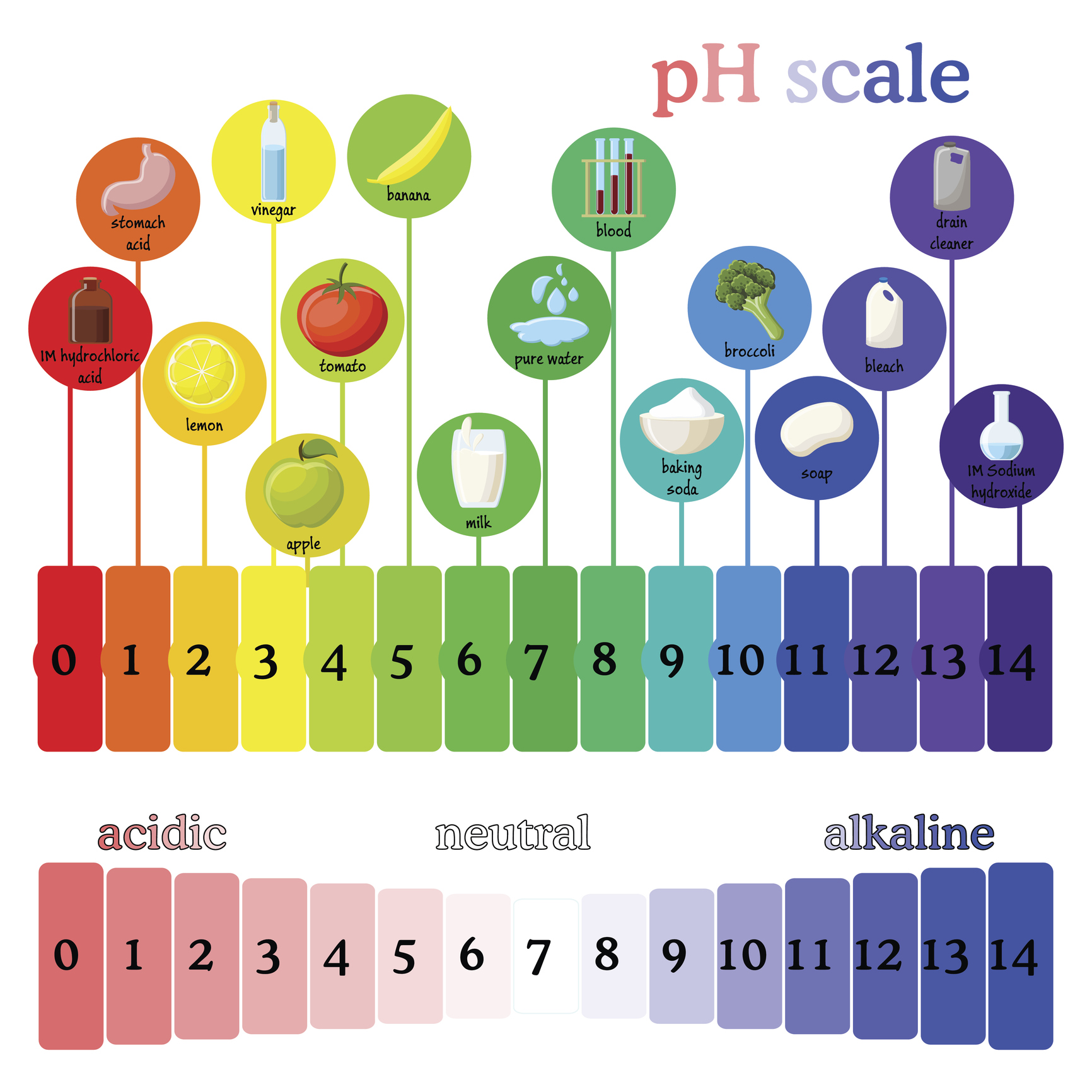
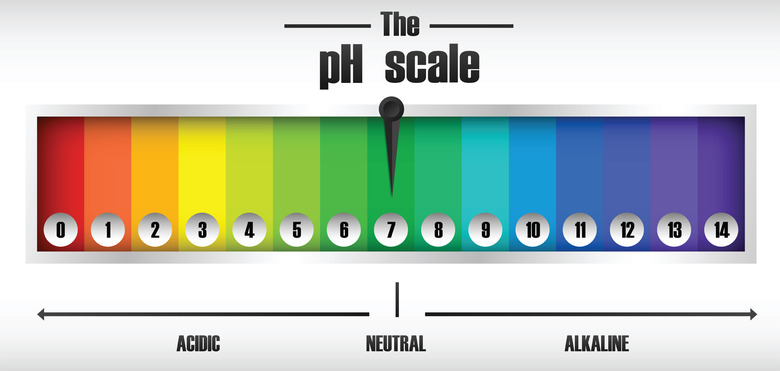
The pH scale, ranging from 0 to 14, provides a numerical measure of a substance’s acidity or alkalinity. A pH of 7 is considered neutral, like pure water. Substances with a pH below 7 are acidic, while those above 7 are alkaline or basic.
The Importance of pH in Everyday Life
The pH of various substances plays a critical role in numerous aspects of our daily lives:
- Food and Beverage: The pH of food influences its taste, texture, and preservation. Citrus fruits, for instance, are acidic, contributing to their tangy flavor. Similarly, the pH of milk influences its curdling process during cheesemaking.
- Cleaning and Hygiene: Cleaning products often rely on pH to effectively remove dirt and grime. Acidic cleaners, such as vinegar, are effective against mineral deposits, while alkaline cleaners, like baking soda, excel at grease removal.
- Personal Care: The pH of our skin and hair is crucial for maintaining their health. Using products with pH levels similar to our skin helps prevent irritation and dryness.
- Gardening: The pH of soil significantly impacts plant growth. Different plants thrive in specific pH ranges, requiring adjustments through soil amendments.
Exploring the pH of Common Household Substances
Understanding the pH of common household substances provides valuable insights into their properties and applications:
Acidic Substances (pH below 7):
- Vinegar (pH 2.4-3.4): A versatile substance used for cleaning, cooking, and preserving food. Its acidity helps dissolve mineral deposits and acts as a natural disinfectant.
- Lemon Juice (pH 2.0-2.4): A natural acid commonly used in cooking and cleaning. Its acidity aids in tenderizing meat, brightening surfaces, and removing stains.
- Coffee (pH 4.5-5.5): The acidity of coffee contributes to its characteristic flavor. However, excessive acidity can lead to heartburn and stomach discomfort.
- Tomatoes (pH 4.0-4.5): The acidity of tomatoes is responsible for their tangy flavor and contributes to their preservation.
- Orange Juice (pH 3.0-4.0): The acidity of orange juice is a source of vitamin C and contributes to its refreshing taste.
Neutral Substances (pH 7):
- Pure Water (pH 7): Water is considered neutral, although its pH can vary slightly depending on dissolved minerals.
- Milk (pH 6.6-6.8): Milk is slightly acidic, but it is often considered neutral for practical purposes.
Alkaline Substances (pH above 7):
- Baking Soda (pH 8.3-8.5): A common household ingredient used for cleaning, baking, and deodorizing. Its alkalinity helps neutralize acids and remove grease.
- Ammonia (pH 11.5-12.5): A strong alkaline cleaner used for disinfecting and removing grease. Its high alkalinity can be corrosive and should be handled with care.
- Drain Cleaner (pH 12-14): Highly alkaline products used to dissolve clogs in drains. Their strong alkalinity can be dangerous and should be used with extreme caution.
- Soap (pH 9-10): Soap is typically alkaline, helping to break down dirt and grease. Its alkalinity can also contribute to dryness, particularly for sensitive skin.
- Bleach (pH 11-13): A powerful alkaline disinfectant used for whitening and sanitizing. Its high alkalinity can damage fabrics and should be used with caution.
Understanding the Impact of pH on Household Activities
The pH of household substances directly influences their effectiveness in various tasks:
- Cleaning: Acidic cleaners, like vinegar, are effective against mineral deposits, while alkaline cleaners, like baking soda, excel at grease removal.
- Cooking: The pH of ingredients influences their taste, texture, and preservation. Acidic ingredients, like lemon juice, help tenderize meat and brighten flavors.
- Gardening: The pH of soil significantly impacts plant growth. Different plants thrive in specific pH ranges, requiring adjustments through soil amendments.
- Personal Care: The pH of skin and hair is crucial for maintaining their health. Using products with pH levels similar to our skin helps prevent irritation and dryness.
The Importance of Safety When Handling Household Chemicals
While understanding pH levels is beneficial, it’s crucial to handle household chemicals with caution. Always follow safety guidelines provided on product labels and avoid mixing different chemicals, as this can lead to dangerous reactions.
FAQs about pH in Household Substances:
Q: How can I determine the pH of a household substance?
A: You can purchase pH test strips or meters specifically designed for home use. These tools provide a quick and easy way to measure the pH of various liquids.
Q: Can I adjust the pH of a substance?
A: Yes, you can adjust the pH of certain substances. For instance, adding vinegar to baking soda will neutralize its alkalinity, resulting in a more neutral pH.
Q: What are the health risks associated with extreme pH levels?
A: Highly acidic or alkaline substances can be corrosive and cause burns if they come into contact with skin or eyes. Ingestion of such substances can lead to serious health problems.
Tips for Safe and Effective Use of Household Substances based on pH:
- Always wear gloves and eye protection when handling strong acids or alkalis.
- Never mix different chemicals without consulting a professional.
- Store chemicals in their original containers, labeled clearly.
- Keep chemicals out of reach of children and pets.
- Use pH-balanced cleaning products for sensitive skin.
- Adjust soil pH based on the needs of your plants.
Conclusion: A Deeper Understanding of pH Enhances Our Daily Lives
Understanding the pH of household substances empowers us to use them effectively and safely. By recognizing the properties of acidic and alkaline substances, we can make informed choices for cleaning, cooking, gardening, and personal care. Armed with this knowledge, we can navigate the chemical world of our homes with greater confidence and awareness.




Closure
Thus, we hope this article has provided valuable insights into The Hidden Language of Household Substances: Understanding pH. We thank you for taking the time to read this article. See you in our next article!