The Hidden Language of Household Liquids: Understanding pH
Related Articles: The Hidden Language of Household Liquids: Understanding pH
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to The Hidden Language of Household Liquids: Understanding pH. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The Hidden Language of Household Liquids: Understanding pH

The world around us is a complex tapestry of chemical interactions, and even the seemingly mundane liquids in our homes play a role in this intricate dance. One key factor that governs their behavior and effectiveness is pH, a measure of acidity or alkalinity. Understanding pH levels allows us to navigate the world of household liquids with greater knowledge and control, ensuring their safe and efficient use.
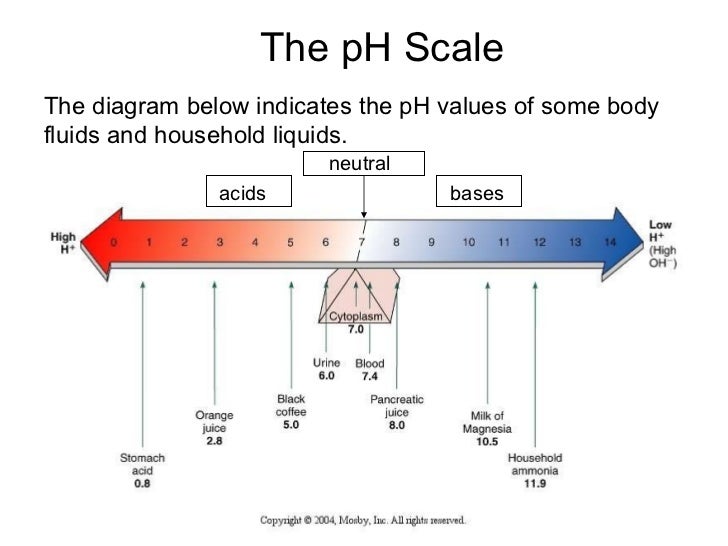
Delving into the pH Scale
The pH scale, ranging from 0 to 14, serves as a numerical representation of the concentration of hydrogen ions (H+) in a solution. A pH of 7 indicates neutrality, while values below 7 signify acidity and values above 7 indicate alkalinity. Each unit on the scale represents a tenfold change in acidity or alkalinity. For instance, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5.
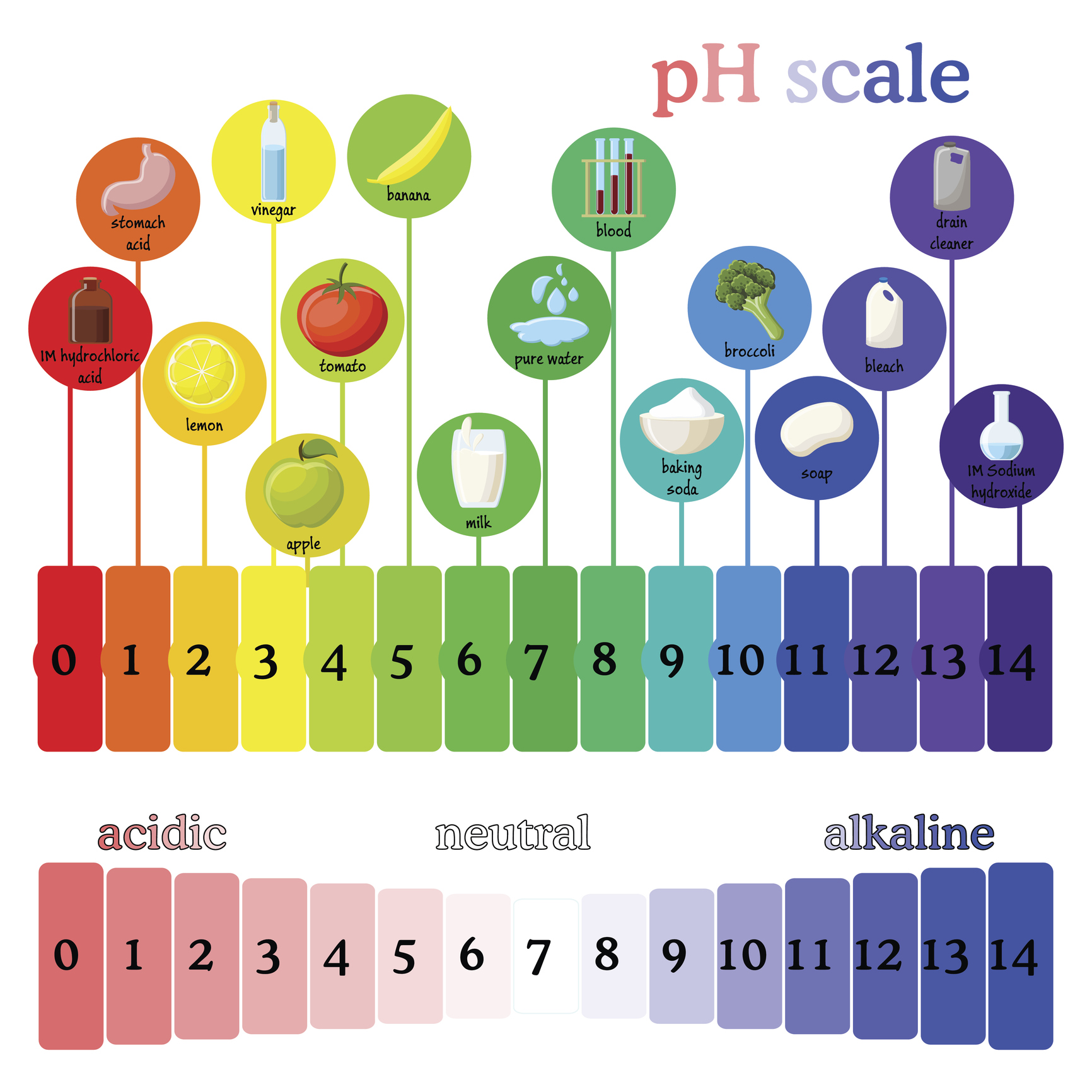
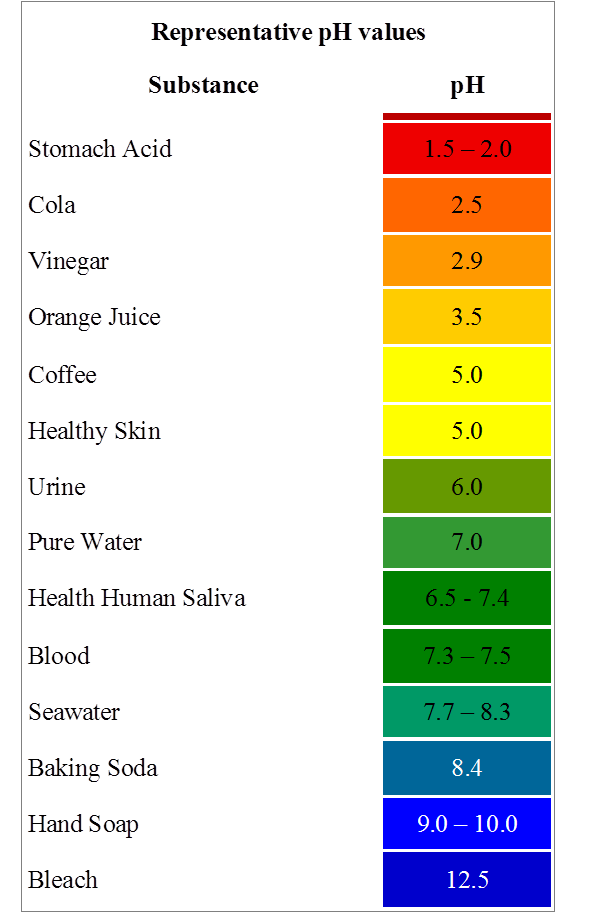
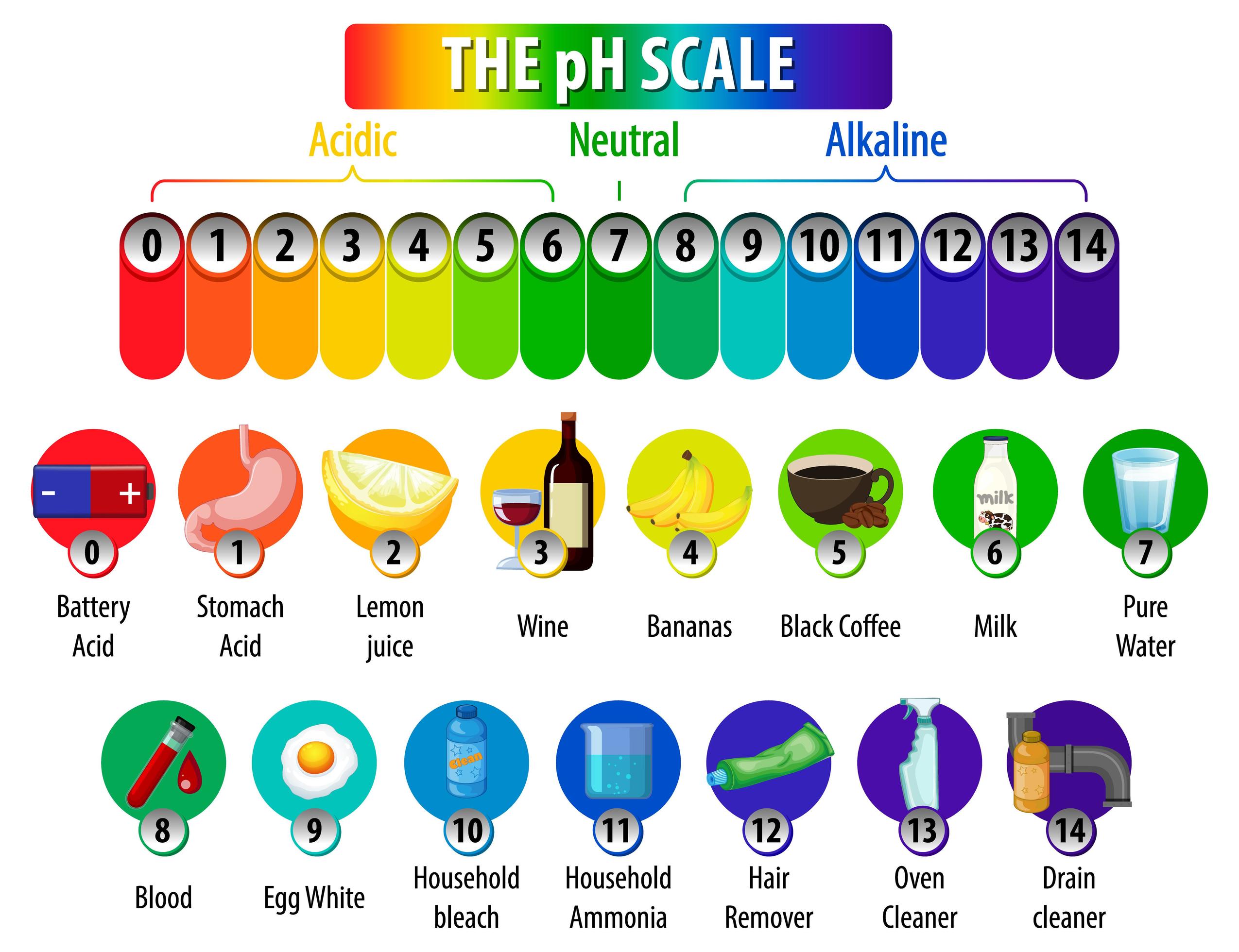
Household Liquids and Their pH Spectrum
The pH of common household liquids varies significantly, reflecting their diverse applications and chemical compositions.
Acids (pH < 7):
- Vinegar (pH 2-3): This common kitchen staple, primarily composed of acetic acid, is a mild acid used for cleaning, deodorizing, and preserving food. Its acidity can help dissolve mineral deposits and neutralize alkaline substances.
- Lemon Juice (pH 2-3): Like vinegar, lemon juice’s acidity stems from citric acid. It finds use in cooking, cleaning, and even as a natural disinfectant.
- Carbonated Beverages (pH 2-4): The fizz in these drinks is due to dissolved carbon dioxide, which forms carbonic acid. Their acidic nature can contribute to tooth enamel erosion if consumed excessively.
- Battery Acid (pH < 1): The highly corrosive sulfuric acid used in lead-acid batteries is a potent acid with a very low pH.
- Stomach Acid (pH 1-3): The hydrochloric acid in our stomachs plays a crucial role in digestion, breaking down food and killing harmful bacteria.
Alkalis (pH > 7):
- Baking Soda (pH 8.5): Sodium bicarbonate, commonly known as baking soda, is a mild alkali used in baking, cleaning, and as an antacid. Its alkaline nature helps neutralize acids and deodorize surfaces.
- Ammonia (pH 11-12): A strong alkali, ammonia is a powerful cleaning agent used for disinfecting and removing grease. However, its strong odor and potential toxicity require careful handling.
- Bleach (pH 11-13): Sodium hypochlorite, the active ingredient in bleach, is a potent alkali used for disinfecting and whitening. Its strong oxidizing properties make it effective against bacteria and stains.
- Drain Cleaner (pH 12-14): These highly alkaline solutions contain strong bases like sodium hydroxide (lye) to dissolve clogs in drains. Their corrosive nature necessitates extreme caution during use.
- Liquid Soap (pH 8-10): Many liquid soaps are slightly alkaline to aid in removing dirt and grease. However, excessively alkaline soaps can irritate sensitive skin.
Neutral Liquids (pH 7):
- Pure Water (pH 7): While the pH of water can fluctuate based on dissolved minerals, pure water is considered neutral.
- Milk (pH 6.5-6.7): Milk’s pH falls slightly below neutral, but it is generally considered close to neutral.
The Significance of pH in Household Liquids
Understanding the pH of household liquids is crucial for several reasons:
- Safety: Highly acidic or alkaline substances can cause skin and eye irritation, burns, and other injuries. Proper handling and dilution, informed by pH knowledge, are essential for safe use.
- Effectiveness: The pH of cleaning solutions influences their effectiveness in removing dirt, grease, and stains. Knowing the pH of specific cleaning products allows for targeted cleaning solutions.
- Compatibility: Mixing certain liquids with different pH levels can lead to unwanted chemical reactions, producing harmful byproducts or reducing the effectiveness of the cleaning agents.
- Health: The pH of food and beverages can influence their taste, texture, and nutritional value. The pH of our bodies is carefully regulated to maintain optimal health, and certain foods and drinks can influence this delicate balance.
FAQs about pH of Household Liquids:
Q: How do I measure the pH of household liquids?
A: pH test strips, available at most drugstores and online, provide a simple and convenient method for measuring pH. These strips change color based on the pH of the solution, allowing you to compare the color to a chart and determine the approximate pH. More precise measurements can be obtained using a pH meter.
Q: How can I safely mix household liquids with different pH levels?
A: It is generally advisable to avoid mixing household liquids with drastically different pH levels unless specifically instructed by the product label. Mixing strong acids and alkalis can generate heat, produce toxic fumes, and even cause explosions.
Q: What happens when I mix vinegar and baking soda?
A: Mixing vinegar (acidic) and baking soda (alkaline) creates a chemical reaction that releases carbon dioxide gas, resulting in fizzing and foaming. This reaction is often used in baking and for cleaning purposes, but it is essential to exercise caution as the reaction can be vigorous.
Q: How can I neutralize a strong acid or alkali spill?
A: In case of a strong acid spill, neutralize it with a weak base like baking soda. For a strong alkali spill, use a weak acid like vinegar. However, always wear appropriate protective gear and use caution.
Tips for Using Household Liquids with Different pH Levels:
- Read Labels Carefully: Always consult the product label for instructions on safe handling, dilution, and mixing.
- Use Protective Gear: Wear gloves and eye protection when handling strong acids or alkalis.
- Store Separately: Store acidic and alkaline liquids separately to prevent accidental mixing.
- Dilute Carefully: Always dilute strong acids and alkalis according to instructions before use.
- Ventilate Well: Use strong acids and alkalis in well-ventilated areas to avoid inhaling fumes.
- Test Before Applying: Test a small, inconspicuous area before applying cleaning solutions to larger surfaces to ensure compatibility.
Conclusion:
Understanding the pH of household liquids is essential for their safe and effective use. From cleaning and cooking to personal hygiene and health, pH plays a critical role in our daily lives. By embracing knowledge about pH, we can navigate the world of household liquids with greater awareness and control, ensuring their safe and beneficial use for ourselves and our homes.


:max_bytes(150000):strip_icc()/__opt__aboutcom__coeus__resources__content_migration__mnn__images__2018__07__pH_scale-6c5cb15299a643d7899c3055bfb9d625.jpg)
Closure
Thus, we hope this article has provided valuable insights into The Hidden Language of Household Liquids: Understanding pH. We hope you find this article informative and beneficial. See you in our next article!
