The Hidden Chemistry of Our Homes: Understanding the pH of Common Household Liquids
Related Articles: The Hidden Chemistry of Our Homes: Understanding the pH of Common Household Liquids
Introduction
With great pleasure, we will explore the intriguing topic related to The Hidden Chemistry of Our Homes: Understanding the pH of Common Household Liquids. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The Hidden Chemistry of Our Homes: Understanding the pH of Common Household Liquids

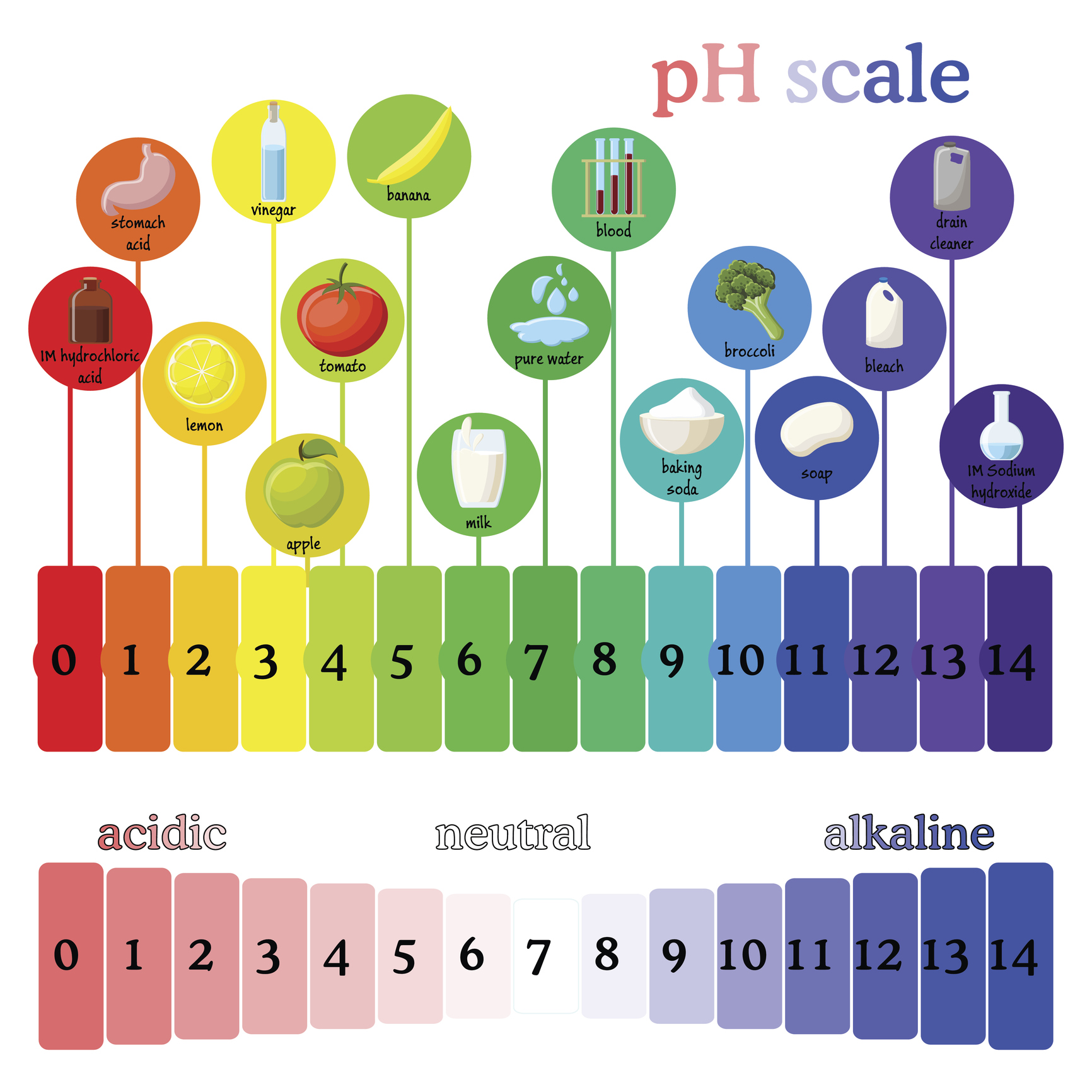
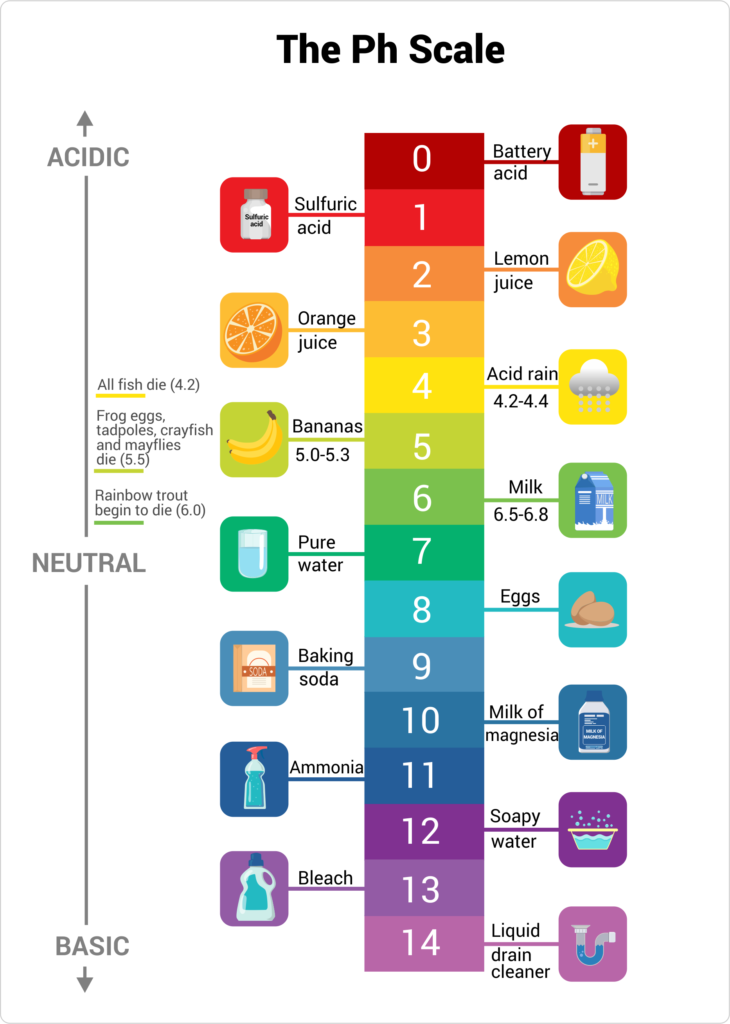
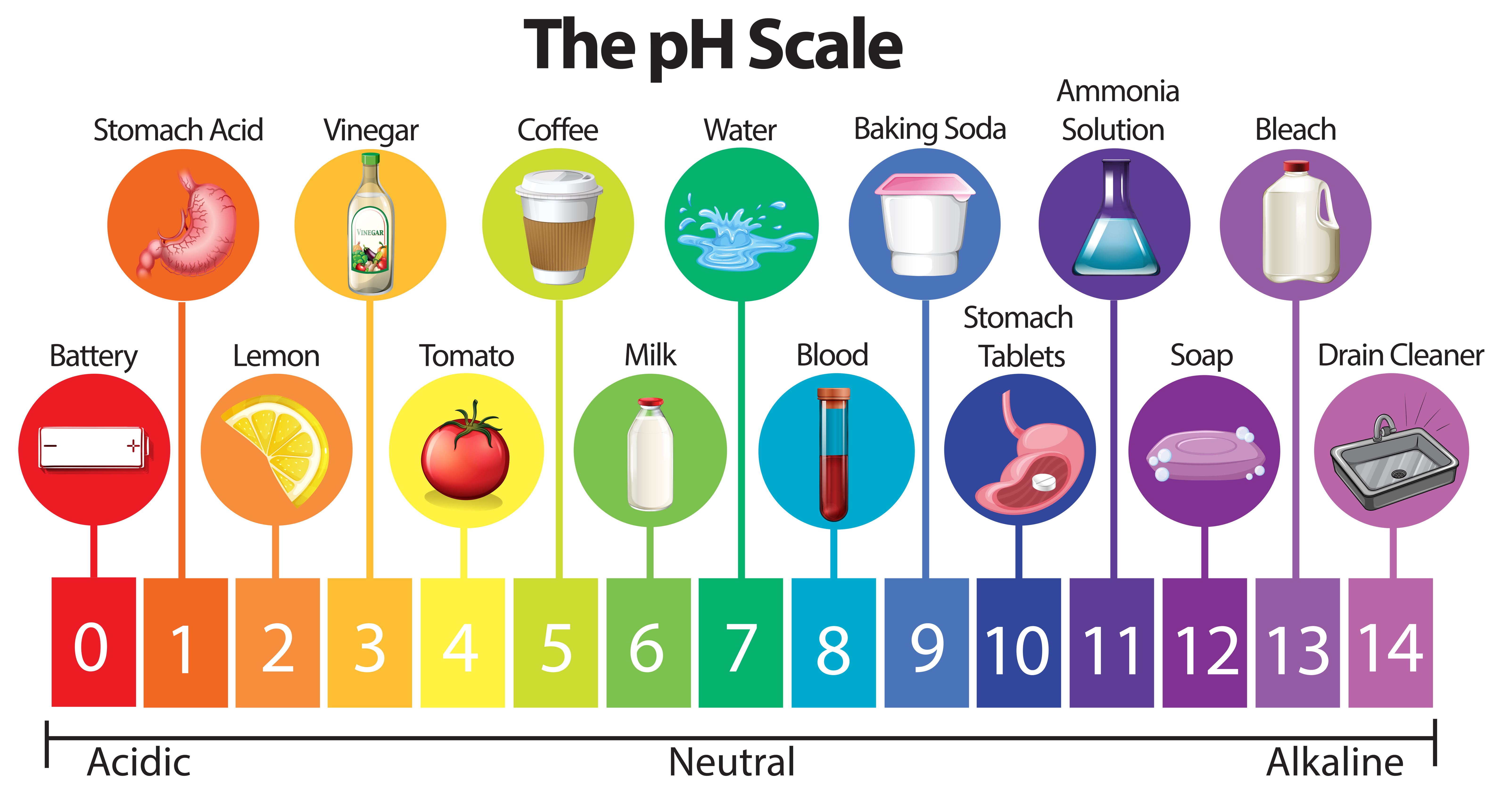
The pH scale, a measure of acidity and alkalinity, is a fundamental concept in chemistry. Its significance extends beyond the laboratory, playing a crucial role in our daily lives, particularly in the context of household products. Understanding the pH of common liquids can help us make informed decisions regarding their use, safety, and effectiveness.
What is pH?
The pH scale ranges from 0 to 14, with 7 representing neutral. Values below 7 indicate acidity, while those above 7 represent alkalinity. Each unit on the scale represents a tenfold change in hydrogen ion concentration. For instance, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4.
The pH of Common Household Liquids:
1. Cleaning Products:
- Vinegar (Acetic Acid): Typically has a pH of 2.4 to 3.4, making it mildly acidic. This acidity is responsible for its cleaning power, effectively dissolving mineral deposits and grime.
- Lemon Juice (Citric Acid): Similar to vinegar, lemon juice boasts a pH of around 2.0 to 3.0. Its acidity contributes to its cleansing properties, also acting as a natural bleaching agent.
- Bleach (Sodium Hypochlorite): A powerful disinfectant, bleach has a pH of 11 to 13, rendering it highly alkaline. This strong alkalinity enables its effectiveness in killing bacteria and viruses.
- Dish Soap: Generally falls in the range of 7 to 10, with some brands exhibiting higher alkalinity. This slightly alkaline nature helps break down grease and food particles.
- Ammonia (Ammonium Hydroxide): A common ingredient in cleaning products, ammonia has a pH of 11 to 12, making it strongly alkaline. This high alkalinity contributes to its effectiveness in removing grease and grime, but also makes it a potential irritant.
2. Beverages:
- Coffee: The pH of coffee varies depending on brewing methods and bean type, but generally falls between 4.5 and 5.5, making it slightly acidic.
- Tea: Similar to coffee, tea’s pH varies based on the type and brewing process. It typically ranges from 5.0 to 6.5, also slightly acidic.
- Soft Drinks: These beverages are highly acidic, with a pH ranging from 2.0 to 3.5. The high acidity can contribute to tooth enamel erosion.
- Fruit Juices: While varying based on the specific fruit, most fruit juices are acidic, with a pH ranging from 3.0 to 4.0.
3. Personal Care Products:
- Shampoo: The pH of shampoo typically ranges from 4.5 to 6.5, making it slightly acidic. This acidity helps to maintain the scalp’s natural pH and prevent dryness.
- Conditioner: Conditioners generally have a slightly higher pH than shampoos, ranging from 5.0 to 7.0, which helps to smooth and soften hair.
- Soap: Soap is typically alkaline, with a pH ranging from 9.0 to 11.0. This alkalinity contributes to its cleansing properties but can be drying for the skin.
- Toothpaste: Toothpaste is designed to be slightly acidic, with a pH ranging from 4.5 to 5.5, to help remove plaque and prevent cavities.
4. Other Household Liquids:
- Milk: The pH of milk is slightly acidic, typically ranging from 6.5 to 6.7.
- Water: Pure water has a neutral pH of 7. However, the pH of tap water can vary depending on the source and treatment processes.
- Honey: Honey is slightly acidic, with a pH ranging from 3.5 to 4.5. This acidity contributes to its antibacterial properties.
Importance of pH in Household Liquids:
Understanding the pH of household liquids is crucial for several reasons:
- Effectiveness: The pH of a cleaning product directly influences its ability to dissolve dirt, grease, and other substances.
- Safety: High acidity or alkalinity can be corrosive or irritating to skin, eyes, and surfaces.
- Compatibility: Mixing products with drastically different pH levels can lead to harmful reactions, producing toxic fumes or reducing effectiveness.
- Environmental Impact: Some cleaning products with high pH levels can contribute to water pollution.
FAQs about pH of Household Liquids:
1. How do I measure the pH of a liquid?
pH can be measured using pH paper, a pH meter, or a pH test strip.
2. Can I adjust the pH of a liquid?
Yes, you can adjust the pH of a liquid by adding an acid or base. For instance, adding baking soda (sodium bicarbonate) to a solution will increase its pH, making it more alkaline.
3. How can I safely use cleaning products with different pH levels?
Always check the product label for safety instructions. Avoid mixing cleaning products with drastically different pH levels, especially bleach with other cleaning agents.
4. What is the ideal pH for drinking water?
The ideal pH for drinking water is slightly acidic, between 6.5 and 8.5.
5. How does pH affect the effectiveness of cleaning products?
The pH of a cleaning product determines its ability to break down dirt, grease, and other substances. For example, a more acidic cleaner will be better at dissolving mineral deposits, while a more alkaline cleaner will be better at removing grease.
Tips for Using Household Liquids with Different pH Levels:
- Always read the product label: Check for safety instructions and recommended dilutions.
- Avoid mixing cleaning products: Mixing products with drastically different pH levels can lead to harmful reactions.
- Use gloves and eye protection: Protect yourself from potential skin and eye irritation.
- Ventilate the area: Ensure proper ventilation when using cleaning products, especially those with strong odors.
- Store cleaning products safely: Keep cleaning products out of reach of children and pets.
Conclusion:
The pH of household liquids plays a significant role in their effectiveness, safety, and environmental impact. Understanding the pH of common liquids can empower us to make informed decisions regarding their use, ensuring both efficient cleaning and a safe environment. By embracing this knowledge, we can navigate the chemical landscape of our homes with greater awareness and responsibility.




Closure
Thus, we hope this article has provided valuable insights into The Hidden Chemistry of Our Homes: Understanding the pH of Common Household Liquids. We appreciate your attention to our article. See you in our next article!