Deciphering the pH Scale: A Guide to Acidity and Alkalinity
Related Articles: Deciphering the pH Scale: A Guide to Acidity and Alkalinity
Introduction
With great pleasure, we will explore the intriguing topic related to Deciphering the pH Scale: A Guide to Acidity and Alkalinity. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Deciphering the pH Scale: A Guide to Acidity and Alkalinity

The pH scale, a seemingly simple numerical system, serves as a powerful tool for understanding the chemical nature of our environment. It quantifies the acidity or alkalinity of a substance, influencing a vast array of processes from biological functions to industrial applications. This article delves into the intricacies of the pH scale, exploring its underlying principles, its wide-ranging applications, and its crucial role in maintaining the delicate balance of our world.
Understanding the pH Scale: A Measure of Hydrogen Ions
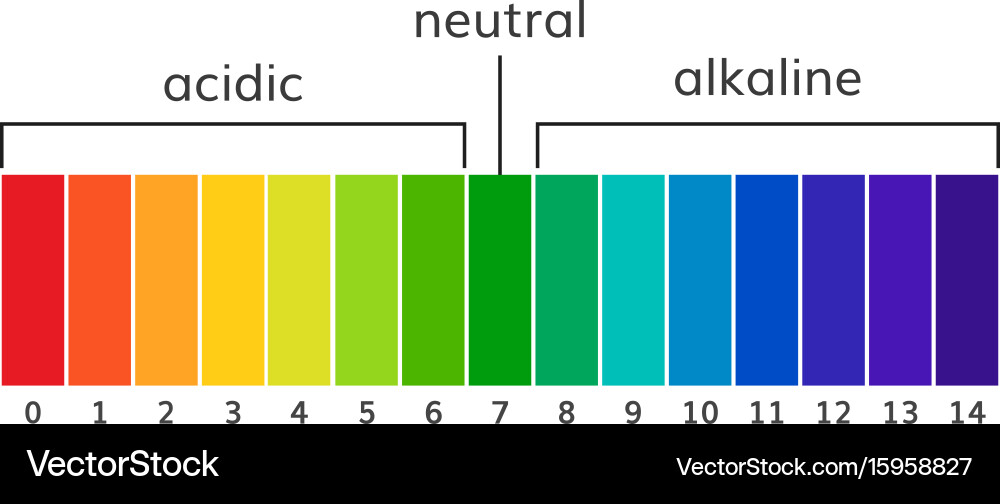
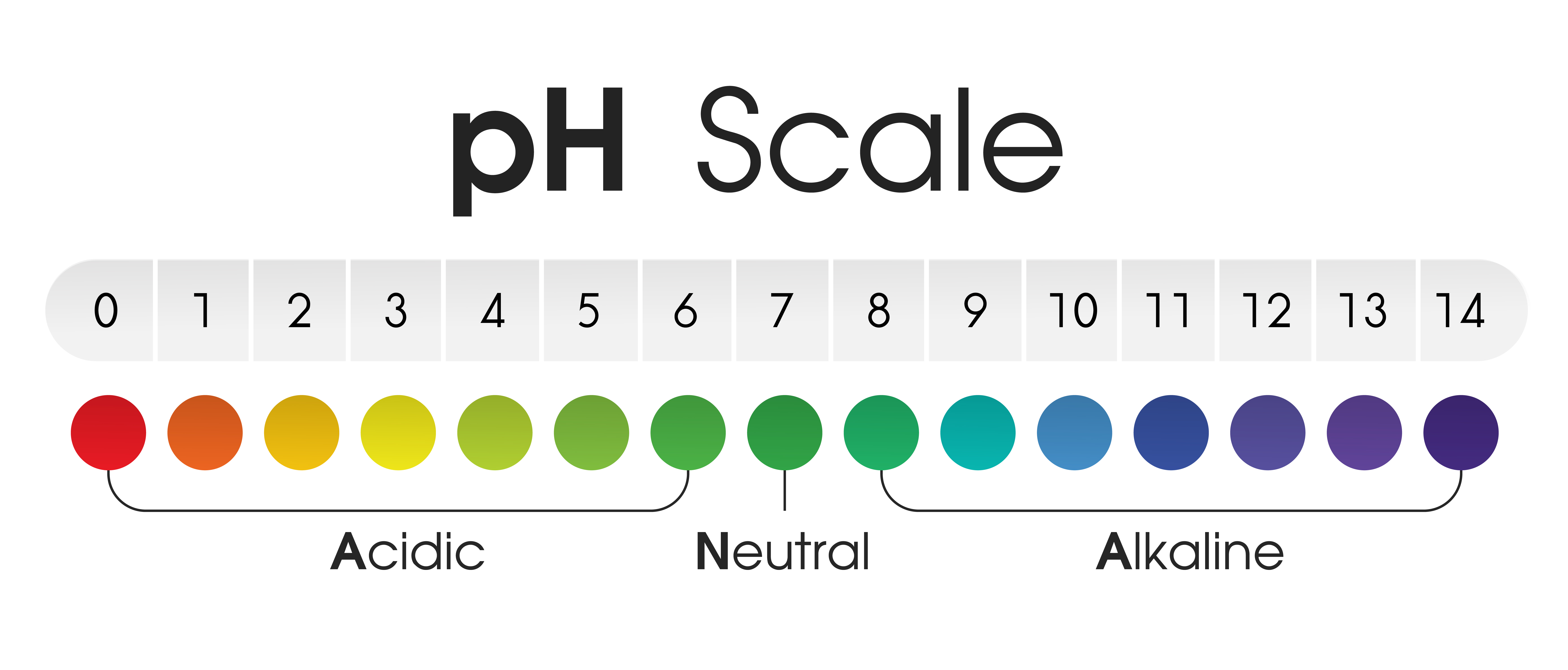
The pH scale, ranging from 0 to 14, is a logarithmic scale that measures the concentration of hydrogen ions (H+) in a solution. A solution with a high concentration of H+ ions is considered acidic, while a solution with a low concentration of H+ ions is considered basic or alkaline.
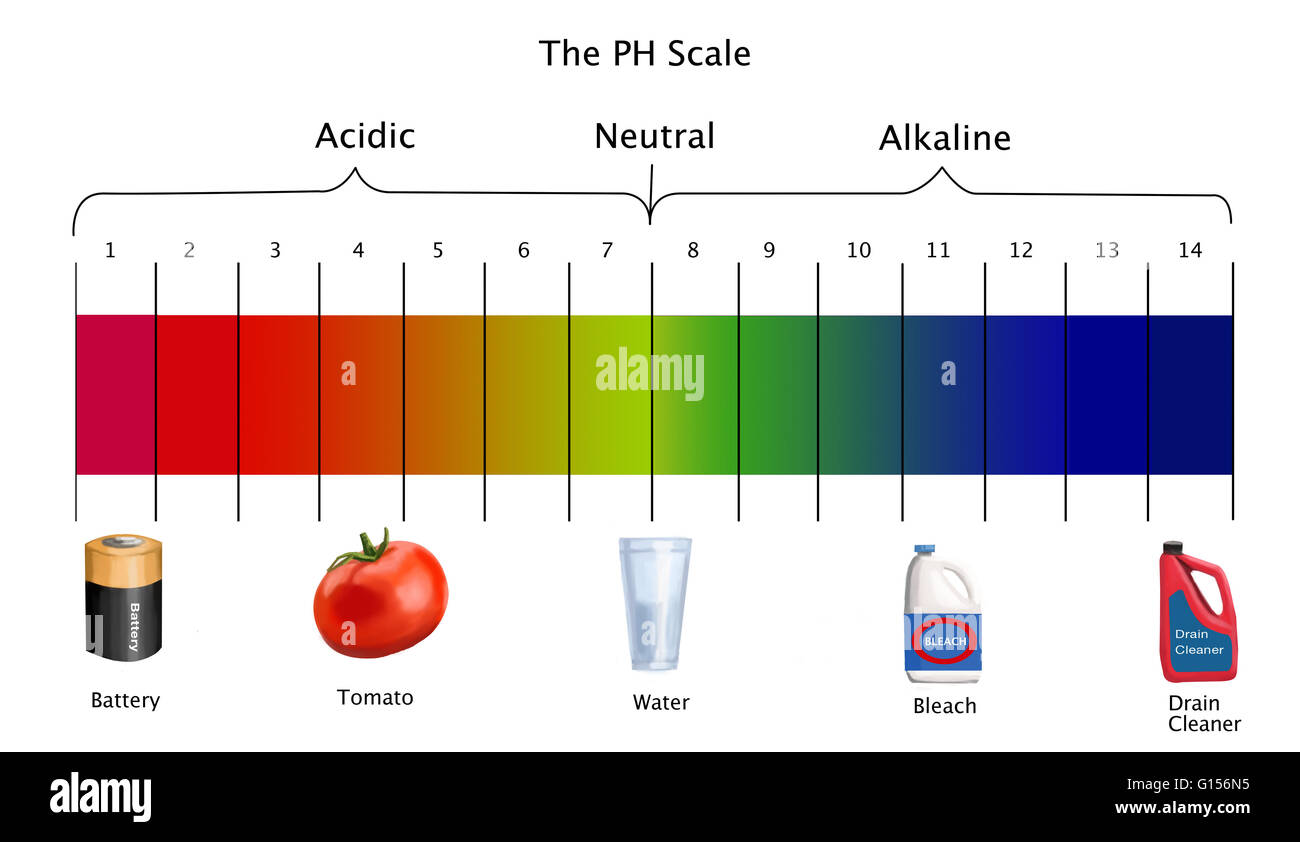
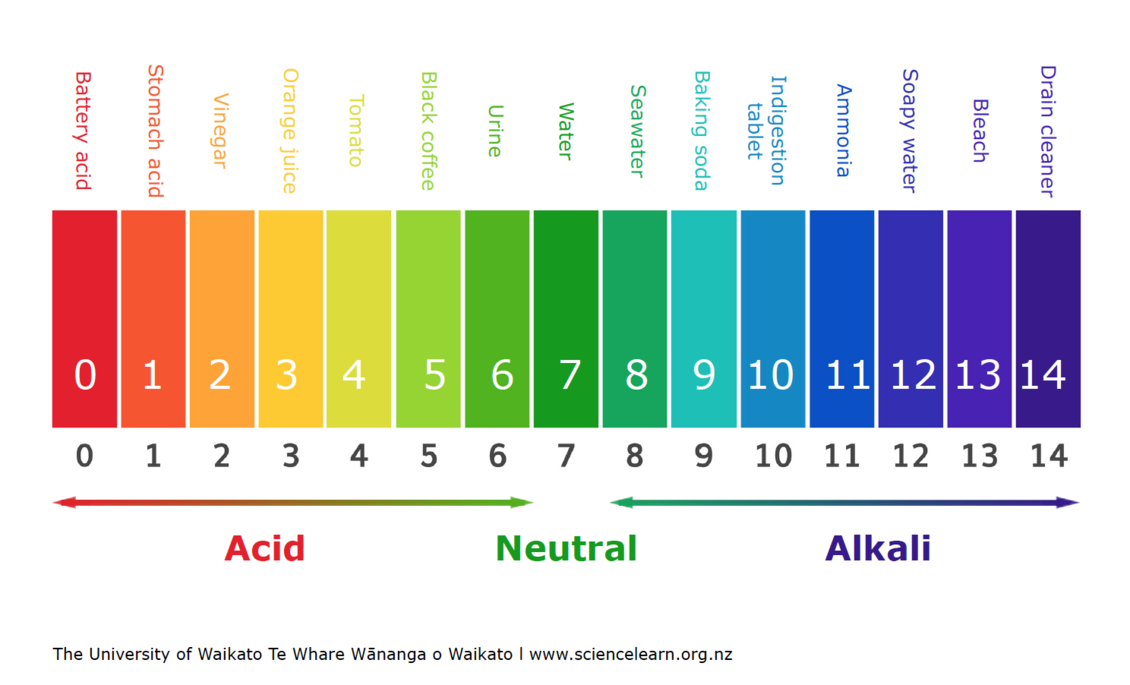
- Acidic Solutions: Solutions with a pH value below 7 are considered acidic. They contain a higher concentration of H+ ions than OH- ions. Examples include lemon juice (pH 2), vinegar (pH 3), and stomach acid (pH 1-2).
- Neutral Solutions: A solution with a pH of 7 is considered neutral. This means that the concentration of H+ ions is equal to the concentration of OH- ions. Pure water is a classic example of a neutral solution.
- Basic or Alkaline Solutions: Solutions with a pH value above 7 are considered basic or alkaline. They contain a lower concentration of H+ ions than OH- ions. Examples include baking soda (pH 9), ammonia (pH 11), and lye (pH 13).
The Importance of pH: A Multifaceted Role
The pH scale transcends mere academic interest, playing a vital role in numerous facets of our existence:
- Biological Processes: The pH of bodily fluids, such as blood, is meticulously regulated to ensure optimal functioning. Even slight deviations can disrupt enzymatic activity, cellular processes, and overall health.
- Agriculture: Soil pH significantly influences plant growth and nutrient availability. Maintaining the appropriate pH range for specific crops is crucial for optimal yield and healthy plant development.
- Industrial Processes: Various industries, including manufacturing, food production, and water treatment, rely heavily on pH control. Precise pH levels are essential for chemical reactions, product quality, and environmental compliance.
- Environmental Protection: Water pH is a critical indicator of water quality and ecosystem health. Maintaining a balanced pH is essential for aquatic life, preventing acidification, and ensuring the integrity of water resources.
Examples of pH in Everyday Life
The pH scale is not confined to scientific laboratories; it permeates our daily lives in countless ways:
- Household Cleaning: Vinegar, with its acidic nature, is a popular household cleaner for removing mineral deposits and grime. Alkaline cleaners, such as baking soda, are effective for neutralizing odors and removing grease.
- Food and Beverages: The pH of food and beverages influences their taste and preservation. For instance, the acidity of citrus fruits contributes to their tangy flavor, while the alkalinity of baking soda helps create fluffy cakes.
- Personal Care: Skin and hair pH are crucial for maintaining their health and appearance. Many personal care products are formulated to balance the pH of skin and scalp.
- Swimming Pools: Maintaining the appropriate pH level in swimming pools is essential for safety and hygiene. A balanced pH prevents skin irritation, reduces the growth of algae, and ensures the effectiveness of chlorine.
FAQs: Addressing Common Queries about the pH Scale
1. How is pH measured?
pH is typically measured using a pH meter, a device that employs an electrode sensitive to hydrogen ion concentration. Alternatively, pH indicator solutions or litmus paper can provide a less precise but readily available method.
2. What are the benefits of pH control?
pH control offers numerous benefits, including:
- Optimizing biological processes: Maintaining the appropriate pH levels for biological systems, such as blood, ensures optimal functioning and health.
- Enhancing agricultural productivity: Controlling soil pH maximizes nutrient availability and promotes plant growth.
- Ensuring product quality: Precise pH control is crucial for industrial processes, ensuring consistent product quality and meeting regulatory standards.
- Protecting the environment: Maintaining balanced pH levels in water bodies protects aquatic life and safeguards ecosystem health.
3. Can pH be changed?
Yes, pH can be changed by adding acids or bases. Acids lower the pH of a solution, while bases increase the pH. The extent of pH change depends on the strength and concentration of the acid or base added.
4. What are the consequences of pH imbalances?
pH imbalances can have various consequences, depending on the context:
- Health problems: pH imbalances in bodily fluids can lead to various health issues, including metabolic disorders and organ dysfunction.
- Decreased agricultural yield: Inadequate soil pH can hinder plant growth, leading to reduced crop yields.
- Product deterioration: pH imbalances can disrupt industrial processes, resulting in product defects or spoilage.
- Environmental damage: Acidification of water bodies can harm aquatic life and disrupt ecosystem balance.
5. How can pH be controlled?
pH control is achieved through various methods, including:
- Adding acids or bases: Adjusting the pH of a solution by adding appropriate acids or bases to neutralize excess acidity or alkalinity.
- Using buffers: Employing buffer solutions to resist pH changes, maintaining a stable pH range.
- Filtering and treatment: Using filtration and treatment processes to remove substances that contribute to pH imbalances.
Tips for Understanding and Using the pH Scale
- Visualize the scale: Imagine the pH scale as a spectrum, with acidic solutions on one end and alkaline solutions on the other.
- Use pH paper: Keep pH paper handy for simple, visual pH assessments of everyday substances.
- Understand the logarithmic nature: Remember that the pH scale is logarithmic, meaning each unit represents a tenfold change in hydrogen ion concentration.
- Consider the context: The significance of pH varies depending on the context. A pH of 7 might be neutral for water but may be detrimental for a biological system.
- Consult professionals: For complex applications or critical situations, consult experts in relevant fields to ensure proper pH management.
Conclusion: The pH Scale – A Vital Tool for Understanding Our World
The pH scale, though seemingly simple, provides a powerful lens through which to understand the chemical nature of our environment. It impacts our health, our food, our industries, and our environment, making it a vital tool for maintaining balance and ensuring the well-being of our planet. By understanding the principles of the pH scale and its applications, we can make informed decisions that promote health, sustainability, and a harmonious relationship with our world.

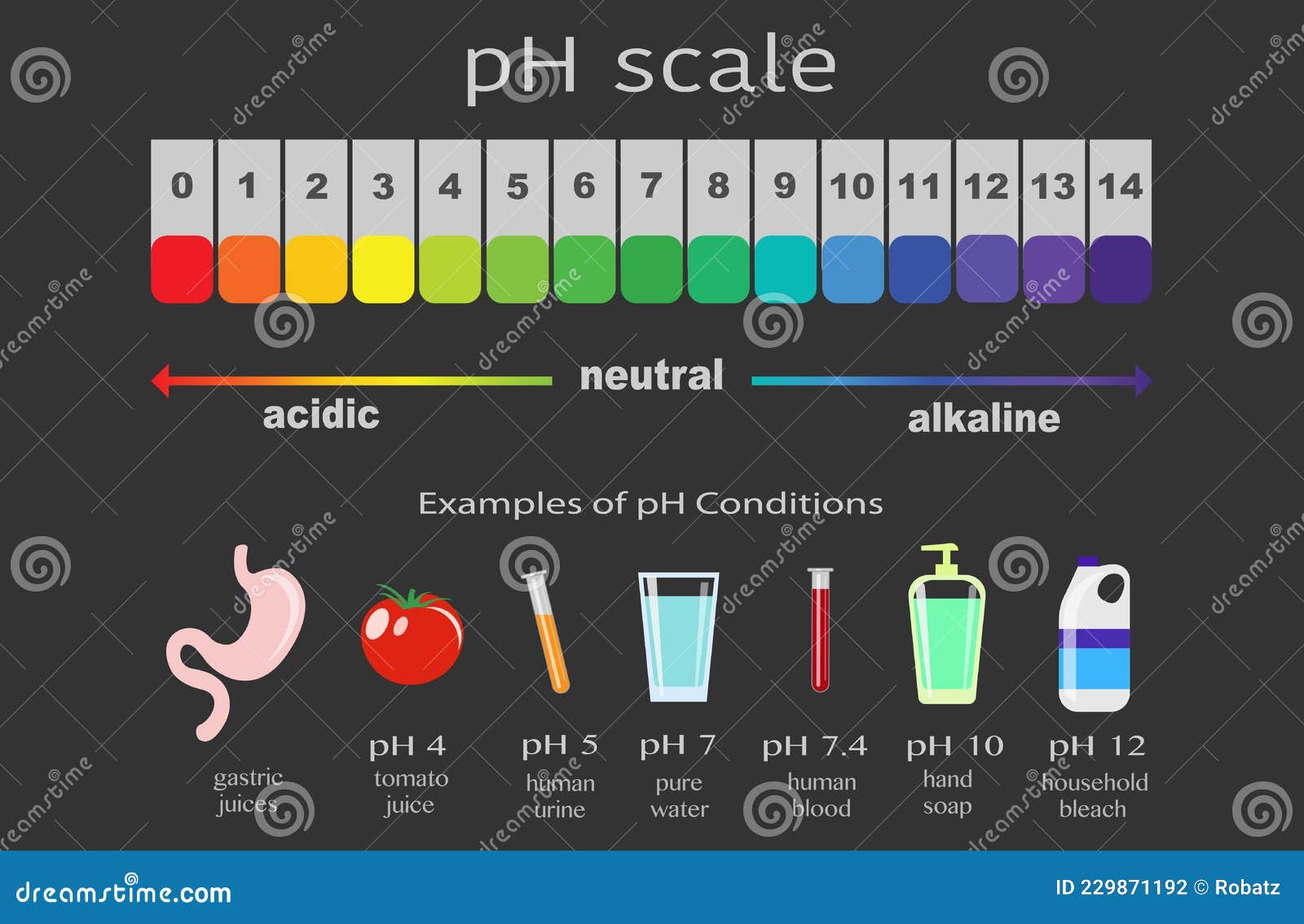

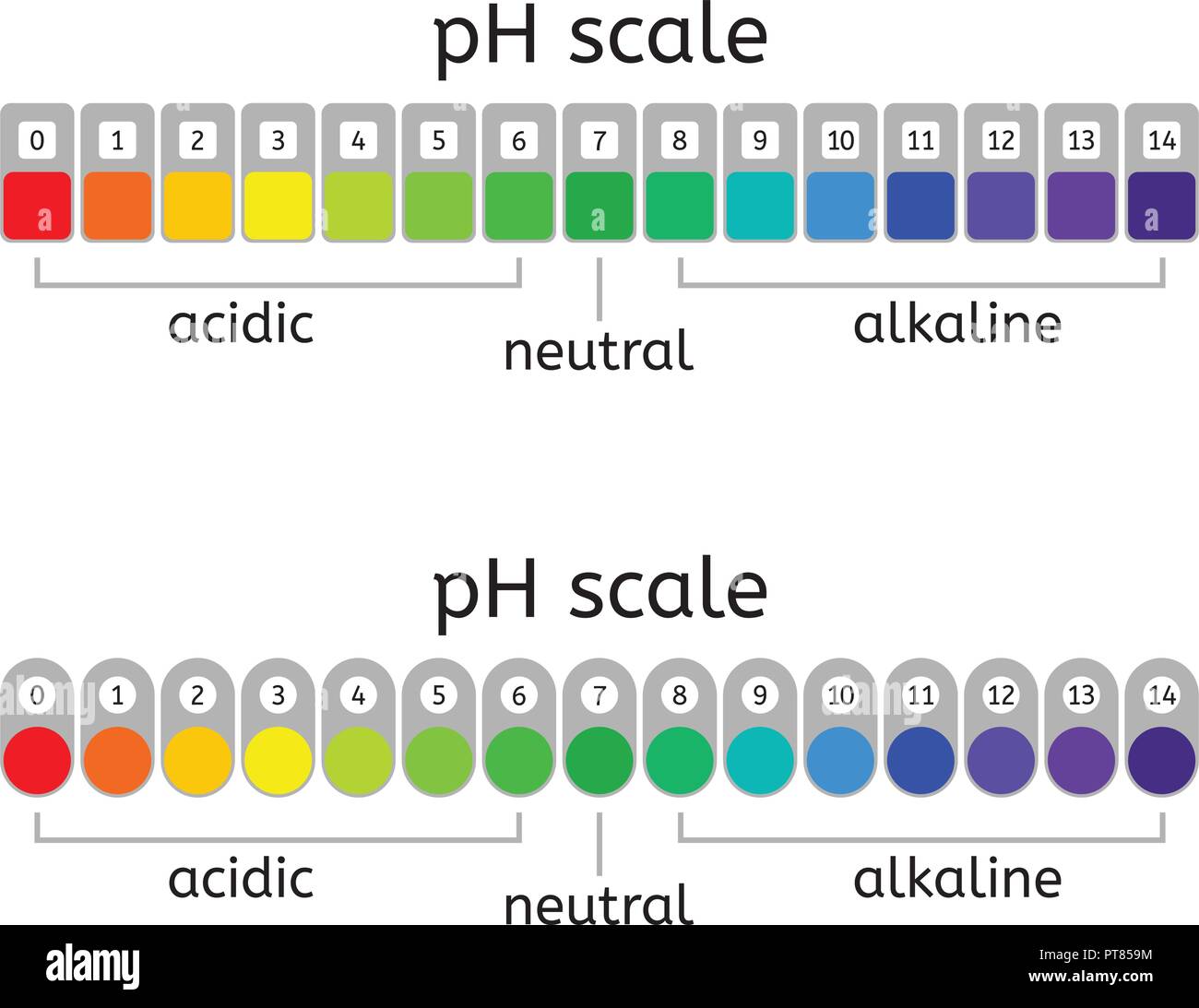
Closure
Thus, we hope this article has provided valuable insights into Deciphering the pH Scale: A Guide to Acidity and Alkalinity. We hope you find this article informative and beneficial. See you in our next article!